Abstract
Introduction
Botulinum toxin is frequently administered serially to maintain therapeutic muscle paralysis, but repeated dose effects on muscle function are largely unknown. This study characterized the muscle response to 2 onabotulinum toxin (BoNT) injections separated by 3 months.
Methods
Animal subjects received a single toxin injection (n=8), 2 BoNT injections separated by 3 months (n= 14), or 1 BoNT and 1 saline injection separated by 3 months (n=8).
Results
The functional effect of 2 serial injections was exponentially greater than the effect of a single injection. While both groups treated with a single BoNT injection had decreased torque in the injected leg by about 50% relative to contralateral legs, the double BoNT injected group had decreased torque by over 95% relative to the pre-injection level. Both single and double BoNT injections produced clear signs of fiber-type grouping.
Discussion
These experiments demonstrate a disproportionately increased effect of a second BoNT injection.
Keywords: muscle physiology, fiber type, denervation, neurotoxin, Botox®
Introduction
Muscle spasticity can result from upper motor neuron lesions, potentially leading to movement impairment and muscle contractures. One of the most widely used pharmacological interventions for spasticity is onabotulinum toxin A [(Botox®); BoNT]. This neurotoxin paralyzes injected muscle by cleaving 1 of the SNARE complex proteins required for synaptic vesicle exocytosis1,2, inhibiting acetylcholine release at the neuromuscular junction. Since the drug’s effect on nerve terminals is reversible3, repeat BoNT injections are necessary to maintain the functional benefit of muscle paralysis.
Despite reports of adequate muscle function after repeated BoNT injections in cerebral palsy treatment4 and other neurological disorders5,6, 2 previous studies observed intramuscular lipid accumulation after multiple BoNT injections, indicating a pathological muscle response7,8. As the BoNT doses used were higher and administered more frequently than usual, it is not clear if these results have direct clinical application. In addition to lipid accumulation, another indicator of normal muscle structure is the typical mosaic pattern of fiber types within the muscles, which indicates a normal innervation pattern. This was not evaluated in either study, so the effect of repeated injections on reinnervation was not explored.
We previously reported the effects of BoNT in a high-resolution functional muscle assay in which the effects of joint manipulation9 and injectate dose and volume10 were investigated. These studies have allowed us to define the “normal” structural and functional response to a single BoNT injection. Given previous reports of pathological response to multiple injections and the general clinical impression that BoNT injection efficacy decreases with time11, we were interested in determining whether repeat injections cause the same effect as the initial injection (i.e., same relative decline in muscle function) or whether a second injection resulted in a decreased or increased response by the muscle. We hypothesized that multiple muscle injections would cause a disproportionately lower response compared to a single injection, since this is the general clinical impression. Thus, the purpose of this study was to quantify muscle functional and structural properties in the adult rat tibialis anterior muscle (TA) in a clinically relevant scenario following 2 injections of BoNT separated by 3 months. For the purposes of this study, “function” refers strictly to the maximum tetanic tension of the skeletal muscle measured as described below in contrast to true patient function reflected by functional activities of daily living.
Material and Methods
Animal Subjects
Laboratory animals used in this study were untrained, mature male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) with an average size of 422.0±7.6g (mean ± standard error of the mean, SEM, n=30). Rats were housed 2 per cage at 20–23°C with a 12h:12h dark-light cycle. The Institutional Animal Care and Use Committee of the Veterans Affairs San Diego Healthcare System approved the experimental methods. After terminal experiments, animals were sacrificed by intracardiac injection of pentobarbital sodium (0.5 mL of 390 mg/mL solution).
Experimental Model
Animal subjects were randomly divided into 3 groups (Fig. 1): a single toxin injection (BoNT; n=8), 2 BoNT injections separated by 3 months (BoNT+BoNT; n= 14), or 1 BoNT and 1 saline injection separated by 3 months (BoNT+Saline; n=8). The latter group was used to insure that a time and age-matched comparison could be made to the BoNT+BoNT group. Contralateral, uninjected hindlimbs were also evaluated. After anesthesia induction (2% isoflurane, 2.0 L/min), rats received a single 100μL injection of BoNT (6.0 units/kg) into the midbelly of the right TA muscle. The muscle midbelly was localized by palpating the largest muscle bulk, and the volume was administered as described previously in detail9. Three months later, animals received 1 of the following: measurement of isometric torque as reported previously12, a second 100μL injection of BoNT, or a 100μL injection of normal saline (Fig. 1). Three months later, isometric torque was measured in the 2 injection groups. Since all animals began the experiment at the same age, the double injection groups were 3 months older than the single injection group at the time of torque measurement.
Figure 1.
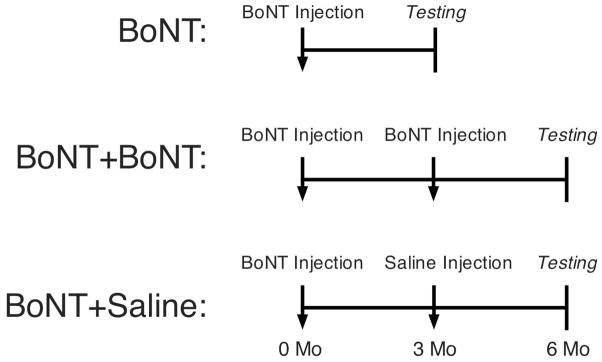
Schematic diagram of experimental procedures. Animal subjects were divided randomly into 3 groups: a single toxin injection (n=8), 2 botulinum toxin injections separated by 3 months (n= 14), or 1 toxin and 1 saline injection separated by 3 months (n=8). The latter group was used to insure that a time and age-matched comparison could be made to the BoNT+BoNT group.
To measure torque, dorsiflexors were activated (15 V stimulus, 650 ms train duration) by stimulation of the common fibular nerve while torque was measured using a custom-designed dynamometer. After activating the muscle over the range 20 Hz to 100 Hz in 20 Hz increments, 3 maximal isometric tetani were elicited at 100 Hz. These 3 contractions were averaged to yield the value for maximal isometric torque, which has a coefficient of variation of about 10%12,13, enabling resolution of small changes in dorsiflexor function. Animals were then sacrificed, and bilateral TA muscles were excised and weighed.
Muscle fiber analysis
Excised TA muscles were snap-frozen in isopentane cooled by liquid nitrogen (−159°C) and stored at −80°C for subsequent analysis as described previously9. Briefly, muscle cross-sections (10 μm thick) were taken from the TA muscle midbelly and treated with 1% BSA and normal goat and rat serum as blocking agents. Sections were incubated overnight with a polyclonal anti-laminin antibody (Sigma, St Louis, MO; dilution 1:1000), and then with the secondary antibody, Alexa Fluor 594 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA; dilution 1:200). The laminin antibody is used to label the fiber perimeter and facilitate fiber area quantification.
Sections were imaged with a SPOT RT digital camera (Diagnostic Instruments, Sterling Heights, MI) on a Nikon Microphot SA epifluorescent microscope (Nikon, Tokyo, Japan) using a 10X objective with a G-2B filter set for red fluorescence. Based on previous sampling studies9, every other field of view from injected muscles was imaged, and 12 images per section were quantified. In contralateral muscles, every third field of view was imaged, and 6 images per section were quantified. Prior to analysis, each image was inspected, and areas with sectioning artifacts, blood vessels, merged fibers, or poor staining quality were omitted. Muscle fiber cross-sectional area was measured using a custom-written macro in ImageJ (NIH, Bethesda, MD).
For quantification of fiber type grouping, we exploited the fact that there are less than 1% type 1 (slow) fibers in the rat TA muscle (see Table 2 of Eng et al. 2010). This number, which ranges from 200–400 fibers/muscle in a normal TA muscle, ensures that almost every type 1 fiber exists as a single cell surrounded by type 2 (fast) fibers. Thus, if fiber-type grouping occurs due to reinnervation of a cluster of fibers, it can be detected morphologically without sophisticated single motor unit experiments. Frozen sections were first treated with 1% BSA and normal horse and rat serum as blocking agents and then incubated overnight with a monoclonal anti-slow myosin heavy chain antibody (WB-MHCs, Vector Laboratories, Burlingame, CA; dilution 1:500). Antibody reactivity was visualized using the indirect immunoperoxidase technique (Vectastain Elite ABC Kit, Vector Laboratories). Fiber type grouping was then quantified by scanning the entire section with a 10X objective through a Leica DFC295 digital camera on a Leica DM6000 B microscope (Leica, Wetzlar, Germany). A “tile scan” was created by stitching together all individual images (ranging from 12–104) using the Leica LAS AF software to create a single large image representing the entire muscle section. Every myosin heavy chain 1 (MyHC1) positive fiber was counted as part of a “group” defined as the total number of fibers touched by another MyHC1 positive fiber. This number ranged from 1 (no grouping) to 24 (severe grouping). For each experimental group, the percentage of fibers in groups of 1, 2, 3, 4, etc. was quantified to yield a fiber-type grouping histogram for that muscle. These values were then binned and averaged across experimental animal subjects.
Intramuscular Lipid Accumulation
Intramuscular lipid accumulation was assessed using Oil Red O staining (American MasterTech, Lodi, CA) of cross-sections obtained from the muscle midbelly. Sections were imaged with the SPOT RT digital camera on a Nikon Microphot SA epifluorescent microscope using a 1X objective.
Myosin heavy chain (MyHC) analysis
MyHC isoform distribution was determined using methods described14. Briefly, a small piece from the muscle midbelly was homogenized, and the myofibril-rich pellets were washed and resuspended in buffers supplemented with protease inhibitor cocktail (5 μL of 100 mM PMSF, 10 μg/μL leupeptin, and 10 μg/μL pepstatin A). Protein was then diluted in sample buffer to a concentration of 0.125 mg/ml.. Separation of MyHC isoforms was performed with SDS-PAGE (16.5 cm by 22 cm, thickness: 0.75 mm) with 22 hrs of migration at 275 V at 4°C. Stacking and resolving gels were 4% and 8% polyacrylamide, respectively. After migration, gels were silver stained according to the manufacturer’s instructions (Bio-Rad, Hercules, CA), and relative isoform content was determined by scanning the gels on a calibrated densitometer (GS-800, Bio-Rad) and performing analysis using Quantity One (Bio-Rad). The positions of MyHC isoforms were determined by their relative electrophoretic mobilities, which have been characterized previously14.
Collagen Content
Traditional methods of expressing “fibrosis” in terms of cross-section area fraction of connective tissue are subject to ambiguity when significant muscle fiber atrophy occurs, as in this model. Thus, to assay for the effect of BoNT on connective tissue, we estimated the amount of extracellular matrix material based on total collagen content. The value was calculated by measuring hydroxyproline content. Briefly, portions of the muscle that contained no internal tendon were isolated and hydrolyzed in 6N HCl at 110°C for 18 hours. After hydrolysis, samples were neutralized and treated with a chloramine T solution for 20 minutes at room temperature followed by a solution of p-diaminobenzaldehyde for 30 minutes at 60°C. Sample absorbance was read from each sample in triplicate at 550 nm and compared to the standard curve to quantify hydroxyproline content. Hydroxyproline content was then converted to collagen content using the constant (7.46) defining the number of hydroxyproline residues in a molecule of collagen.
Statistical analysis
Experimental results were analyzed by 2-way analysis of variance using treatment group and side as grouping factors. Post-hoc Bonferroni tests were used to perform multiple paired comparisons between pairs of groups. All results are reported as mean ± SEM unless otherwise noted.
Results
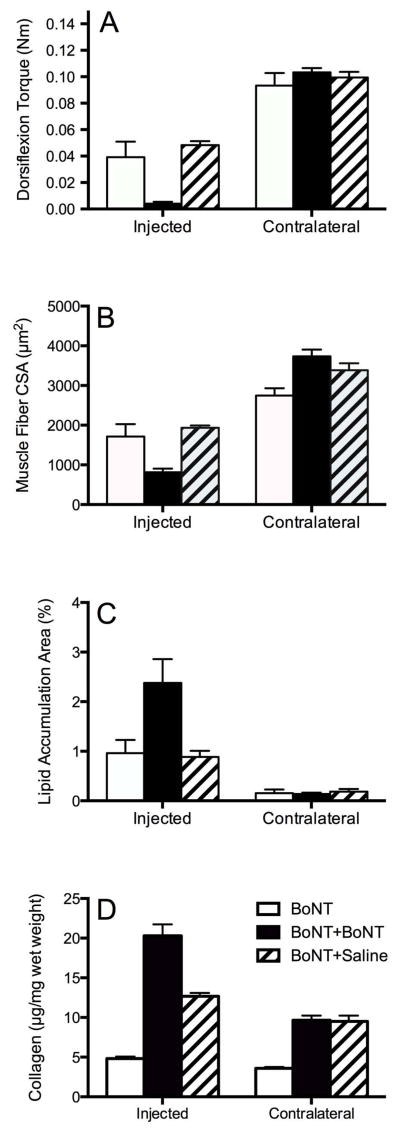
The functional muscle effect of serial BoNT injections was quite different from the effect of a single injection on both the absolute and relative scales. This was indicated statistically by the 2-way ANOVA of dorsiflexion torque, which revealed a significant effect of side (injected vs. contralateral, P<0.001), a significant effect of treatment (among the 3 experimental groups; P<0.001), and a significant side x treatment interaction (P<0.001; Fig. 2A). There was no significant difference between injected legs given either a single BoNT dose or a single BoNT dose followed by a single saline dose (P>0.6; compare open and hatched bars in Fig. 2A), indicating that the 3 month age difference did not affect dorsiflexion torque. However, the experimental leg treated with 2 serial BoNT doses was significantly weaker compared to the other treatments (P<0.001; Fig. 2A). Specifically, while both legs injected with a single BoNT injection only generated about 50% of the dorsiflexion torque of the contralateral leg after 3 months, the double BoNT injected leg remained essentially completely paralyzed (torque decreased by over 95% relative to BoNT or BoNT+Saline groups). Thus, the second BoNT injection was more effective (greater decrement in muscle function and more persistent) compared to the first injection. This result was roughly but not perfectly paralleled by changes measured in skeletal muscle mass (Table 1).
Figure 2.
(A) Torque measured from injected and contralateral hindlimbs. Note a reduction in torque in all BoNT injected limbs compared to contralateral uninjected muscle (P<0.00001). In the injected limbs, torque was reduced significantly in the double BoNT injected group compared to other groups (P<0.0001). (B) Average muscle fiber CSA measured in injected BoNT hindlimb and contralateral uninjected side. There was a significant decrease in fiber CSA in double BoNT injected muscle compared to single injected muscle, in BoNT followed by saline injected muscle (P<0.0001), and in all injected muscles when compared to contralateral uninjected muscles (P ≤ 0.05). (C) Percentage of lipid accumulation area in injected and contralateral uninjected TA muscles. Note a higher percentage of lipid accumulation in double BoNT injected muscles compared to contralateral (P<0.0001). (D) Collagen content of the experimental groups measured by hydroxyproline content. Collagen content increases with both double injection groups. This appears to be more of an effect of age than neurotoxin injection, since the second saline injection elicited the same response.
Table 1.
Injected and Contralateral Tibialis Anterior Muscle Masses (g)
| Group | Injected | Contralateral |
|---|---|---|
| BoNT | 0.74 ± 0.04+ | 1.05 ± 0.02* |
| BoNT + BoNT | 0.31± 0.01# | 1.05 ± 0.01* |
| BoNT + Saline | 0.62± 0.02 | 0.99 ± 0.03* |
N= 8–14/group (See Methods). Values represent mean ± SEM.
when compared to injected muscle (P<0.0001);
when compared to BoNT and BoNT +Saline groups (P<0.0001);
when compared to BoNT +Saline group
Structural parameters qualitatively paralleled functional results. For example, muscle fiber cross-sectional area, a surrogate for the total amount of contractile material present, showed the same 2-way ANOVA results (significant effect of side, P<0.001; significant effect of treatment, P<0.05; significant side x treatment interaction, P<0.001; Fig. 2B). While muscle fiber size decreased by about 35% for the singly injected experimental legs relative to contralateral legs, the double BoNT injection decreased fiber size by over 75%. In other words, the second dose had a greater effect compared to the first.
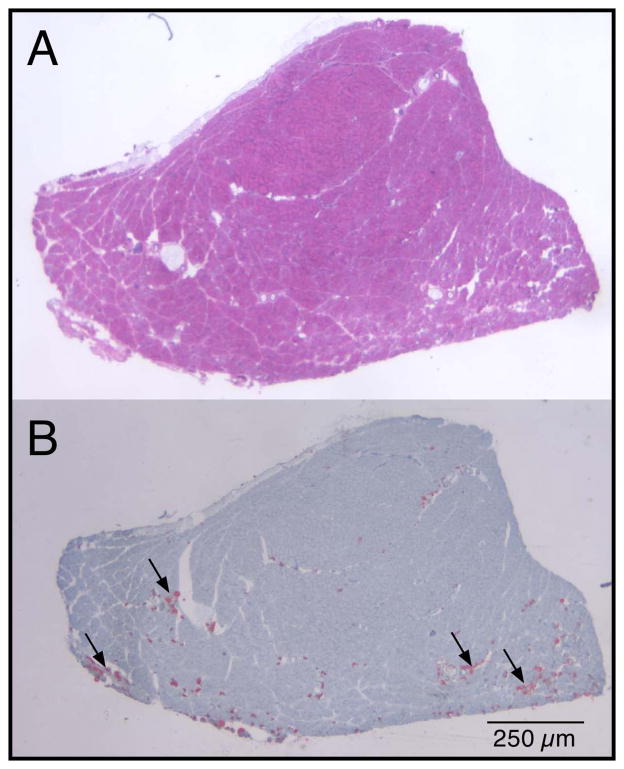
A very small amount of lipid was measured in the injected muscles of all groups (~1–3% relative to total muscle cross-sectional area). Thus, this particular injection paradigm did not elicit a dramatic lipogenic response, but the amount of lipid was, nevertheless, abnormal. Noninjected muscles show virtually no lipid (<0.2%). Two-way ANOVA revealed a significant effect of side (P<0.001), a significant effect of treatment (P<0.05), and a significant side x treatment interaction (P<0.05; Fig. 2C). While both single injection experimental muscles showed about a 7-fold increased lipid accumulation, the double injected muscle showed over a 12-fold increase. Morphological appearance of lipid was relatively superficial in the muscle belly and appeared to collect in clusters in the perimysial space, but not necessarily in areas containing the most significantly atrophied fibers (arrows, Fig. 3).
Figure 3.
TA cross-section from a BoNT injected muscle. (A) Traditional hematoxyln and eosin reflects general morphology. (B) Oil Red O staining reveals red droplets that represent fat accumulation, which was not observed in any control muscles.
Myosin heavy chain composition reflects the overall level of muscle use. Muscles that experience increased levels of use tend to increase their type 1 fiber type percentage, and muscles with decreased level of use have increased type 2 fiber type percentage15. Fiber subtypes can also shift within the fast fiber type from 2Ato 2X to 2B (slowest to fastest) as muscle use decreases. The myosin heavy chain profiles of all injected muscles shifted slightly but significantly toward the slower phenotype with a significant decrease in type 2B and significant increase in type 1 and type 2A fiber type percentage (Table 2). The double injected leg increased in this direction to a significantly greater extent compared to either singly injected leg (P<0.05). Neither a side nor treatment effect was observed for type 2X fibers, but this was expected since, in the process of transformation from type 2B to type 1, only the extreme fiber types typically demonstrate significant changes16.
Table 2.
Myosin heavy chain distribution (%)
| Group | MyHC1 | MyHC2A | MyHC2X | MyHC2B | ||||
|---|---|---|---|---|---|---|---|---|
| Injected | Contralateral | Injected | Contralateral | Injected | Contralateral | Injected | Contralateral | |
| BoNT | 4.8±0.5 | 1.7±0.3* | 15.3±2.0 | 7.6±0.9* | 50.3± 2.0 | 43.0±3.9 | 29.8±3.0 | 47.5±4.6* |
| BoNT + BoNT | 8.6±0.8# | 1.5±0.2* | 28.7±1.7# | 4.8±1.5* | 44.3±1.2 | 46.0±2.8 | 18.3±1.9 | 47.5±2.4* |
| BoNT + Saline | 3.8±0.6 | 1.0±0.1* | 15.3±1.2 | 7.5±0.6* | 54.1±2.6 | 44.5±4.0 | 26.7±2.6 | 47.0±4.0* |
N= 8–14/group (See Methods). Values represent mean ± SEM.
when compared to injected muscle (P<0.05);
when compared to BoNT and BoNT +Saline (P<0.0001).
Abbreviations: MyHCx, myosin heavy chain type “X.”
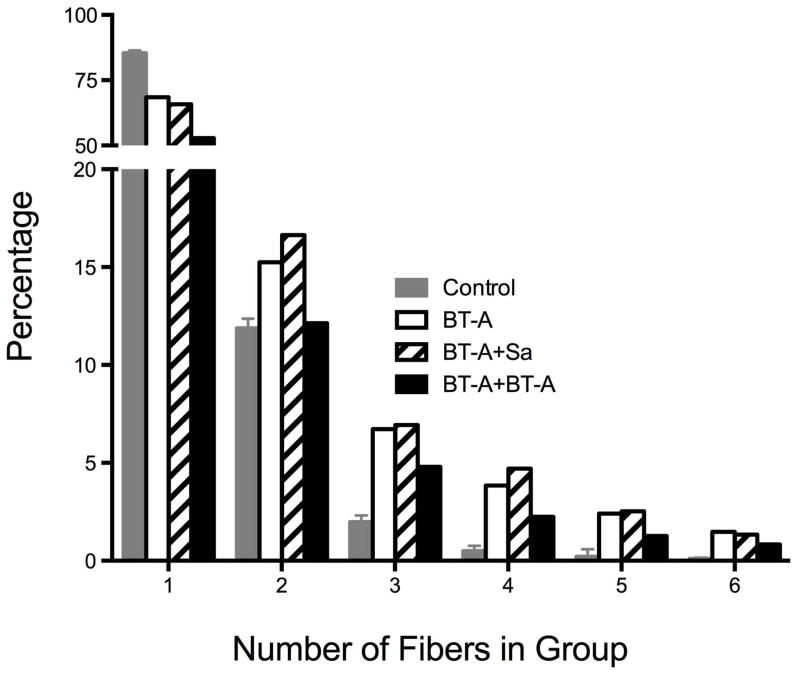
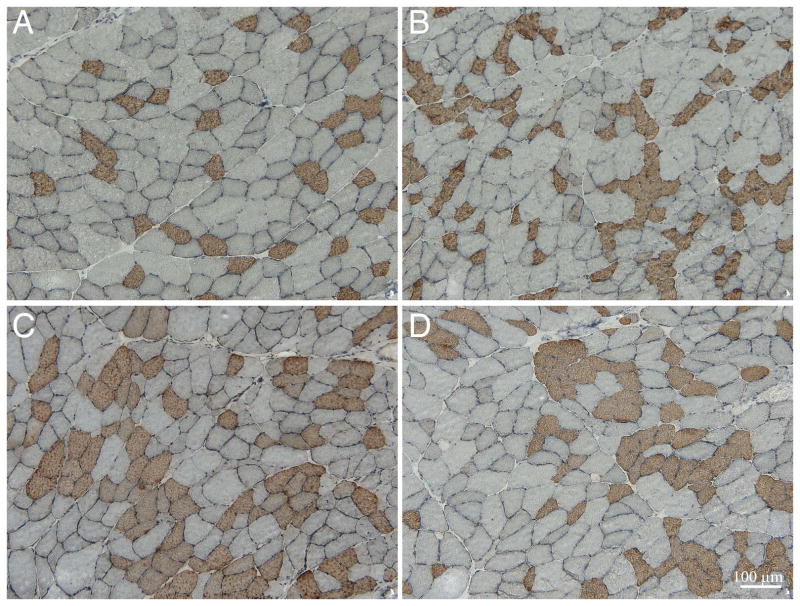
Both single and double BoNT injection produced signs of fiber type grouping. In control skeletal muscles, 85±1% of type 1 fibers were completely surrounded by type 2 fibers (Fig. 4, open bar, number of fibers in “group” equals 1) while in all 3 of the BoNT groups, less than 70% of type 1 fibers were completely surrounded by type 2 fibers (P<0.001; Fig. 4, black, hatched, and grey bars). Fiber type grouping was obvious in all of the BoNT injected groups (note significant differences between control fibers and all other groups with fiber clusters of 2–6) with maximum numbers of fibers in a cluster ranging from 16–24, while control tissue had a maximum of 6 fibers in a group, as was evident visually on the micrographs (Fig. 5).
Figure 4.
Histogram of percentage of type 1 fibers surrounded by type 2 fibers. Note that in control skeletal muscles, 85±1% of type 1 fibers were completely surrounded by type 2 fibers (i.e., number of fibers = 1) while in all 3 of the BoNT groups, less than 70% of type 1 fibers were completely surrounded by type 2 fibers (P<0.001). Data are shown only for “groups” up to 6 fibers, which was the limit of control muscles. Several BoNT injected muscles contained fiber groups of over 20 fibers.
Figure 5.
Representative immunohistochemical images from (A) contralateral control of BoNT +Saline injected, (B) BoNT injected, (C) BoNT + BoNT, or (D) BoNT +Saline groups. All sections were labeled with a primary antibody against type 1 myosin heavy chain. Fiber type grouping was observed in all BoNT injected muscles, with groups ranging from 16–24 fibers. Scale bar: 100μm.
Collagen Content
Collagen content in the BoNT double-injected group was higher compared to either the single BoNT injection group or contralateral muscles (P<0.0001). This was demonstrated statistically by the 2-way ANOVA of collagen content, which revealed a significant effect of side (injection vs. contralateral, P<0.0001), a significant effect of treatment (among the 3 experimental groups; P<0.0001), and a significant side x treatment interaction (P<0.0001; Fig. 2D).
Discussion
The purpose of this study was to determine the effect of a second BoNT injection into a muscle that had been injected previously. We hypothesized that the second dose would have a relatively smaller functional effect based on the literature and clinical experience, but this hypothesis was soundly rejected based on our functional and structural measurements.
It is interesting that numerous studies have shown both safety and efficacy of repeated BoNT injections to reduce spasticity, improve muscle tone, and functional mobility6. In these studies, patients received 3 to 5 injections of BoNT, and a minimum of 3 months elapsed between treatment cycles. Subsequent injections were performed when deemed clinically necessary. Our results suggest that a subsequent injection actually has a much greater relative effect on the muscle compared to the initial injection, at least in normal muscle. In functional terms, we found that, while the initial injection decreased torque by about 50% 3 months post injection, the subsequent injection almost completely eliminated muscle function, decreasing torque by about 95% (Fig. 2A). This appears to be due to the fact that the muscle had not recovered by the 3-month period, even though this is the period suggested from clinical experience. We suspect and have previously reported9,10, that a relatively low level of muscle force production can still result in enough function to produce a functional effect that would result in the need for treatment. Thus, even after 3 months, when muscle function is about half of normal, spastic activity of this muscle may still create joint deformity requiring treatment. We thus do not interpret our animal model results as contradicting clinical experience. Rather, we believe these results uncover a previously unappreciated phenomenon. When muscles are injected with BoNT prior to complete recovery, the subsequent BoNT effect is much stronger compared to the initial effect. We injected 6U/kg at both time intervals, but, based on our understanding of the relationship between dose and efficacy in this animal model (c.f. Fig. 1A of Hulst et al. 2013), we suggest that a dose of less than half may be as efficacious as the initial injection. Clearly our results suggest that even when injections are separated by 3 months, the muscle has not fully recovered in terms of either structure (Figs. 2B and 2C) or function (Fig. 2A). Again, this appears to conflict with traditional clinical wisdom which suggests that higher doses are required upon subsequent injections. It should be noted that our studies were performed in normal rat muscle, and therapeutic use of BoNT is typically performed on muscles with neuromuscular abnormalities, so it is not clear how well our results apply to abnormal muscle.
As with our previous studies9,10, there was a highly significant relationship between muscle fiber cross-sectional area and joint torque in all muscles studied (data not shown). Linear regression between muscle fiber CSA and joint torque revealed a highly significant result (P<0.0001) with the relationship described by the equation:
This is nearly the identical regression relationship reported previously10. The significance of this finding is that muscle fiber area appears to be the primary factor producing joint torque at both the 3- and 6-month post-injection time points. The BoNT-induced muscle fiber atrophy, secondary to interruption of neuromuscular junction activity appears to be responsible for the muscle functional deficit. This is consistent with observed changes in human skeletal muscle after BoNT injection.17 Since the torque-muscle fiber area relationship still holds after a second injection, we suggest that the neuromuscular junctions are “weakened” by the initial injection so that subsequent injections disrupt the NMJ interface to a much greater extent compared to when the NMJ is healthy.
Increased collagen content
We measured “fibrosis” indirectly using the hydroxyproline assay, since alterations in fiber size that occur in atrophy models often appear as fibrotic when actually the decreased fiber size allows expansion of the extracellular space. While the collagen content increases observed in injected legs were expected, contralateral increases were not. Our interpretation is that BoNT-induced muscle fiber atrophy and recovery leads to collagen deposition resulting in increased collagen content in the 2 injected muscles. However, the BoNT+BoNT and BoNT+Saline groups are also 3 months older than the single BoNT group. There is ample evidence that age and connective tissue content are correlatated positively, which leads us to interpret the contralateral effect as an age effect.
Lipid accumulation
This report of increased lipid accumulation after BoNT injection is consistent with previous reports in both rabbit and mouse models, thus it does not appear that this result is species-specific7,8. In these previous studies, the total unit of injection was 21U/kg in rabbits over a period of 6 months7 and approximately 0.6U/g in mice over a 56 day period8. Lipid accumulation in those studies was qualitatively much more severe (but was not quantified), so there does seem to be a cumulative dose-response relationship. We do not believe that the increased intramuscular lipid accumulation is caused by a direct effect of BoNT; rather, it appears to be an indirect result of neuromuscular junction (NMJ) paralysis. This theory is based on previous studies that have demonstrated accumulation of intramuscular lipid after denervation18,19, and that small BoNT doses do not result in lipid accumulation10.
The mechanisms involved in intramuscular lipid accumulation are not completely known, but it has been hypothesized that satellite cells themselves have adipogenic potential20. It is also possible that the recently described “fibro-adipogenic progenitors” identified in skeletal muscle21,22 are directly or indirectly activated by BoNT treatment. Future studies using lineage tracing are required to determine the precise source of intracellular lipid deposition and its relationship to BoNT injection. It is also possible that muscle paralysis may lead to increased storage of energy-producing substrates. Lipid is the main energetic substrate of type 1 and type 2A fibers, and the double injected rat TA muscle has nearly 50% of these fiber types (Table 2), compared to less than 5% in the normal rat TA23. A previous report demonstrating a significant increase in lipoprotein lipase (an enzyme that facilitates transport of free fatty acids into muscle) after BoNT injection may be causally related to the lipid accumulation24. Of course, it is also possible that an increase in type 1 and type 2A fibers, along with increased lipid actually results in a less-fatigable muscle. This result that might be clinically preferable, since it has been reported that children with cerebral palsy suffer from increased muscle fatigue,25 but this observation is not universal.26
Muscle fiber type transition
It is well known that skeletal muscle fibers are dynamic structures capable of altering their phenotypes due to altered neuromuscular activity27. In general, transition toward the faster muscle fiber phenotype is observed after decreased muscle use, such as immobilization, spinal cord injury, and weightlessness, while transition toward the slower fiber phenotype is observed with increased loading (exercise, chronic stretch, functional overload, and hypertrophy)15. Although BoNT injection caused temporary muscle denervation and resulting decreased neuromuscular activity, we measured a higher expression of type 1 (slow muscle fibers) and type 2A (fast oxidative muscle fibers) myosin heavy chains in injected muscles, which is opposite to results from other disuse models but is in agreement with previous studies in the rat gastrocnemius, plantaris28, laryngeal29, and extraocular muscles30 after BoNT injection. This result reinforces the idea that denervation and, in this case, chemodenervation, elicits a muscle response that is not simply related to muscle “use level” but may also be related to NMJ activity and/or neurotrophic factors.
In terms of fiber type distribution, muscle cross-sections are described as having a mosaic pattern of fiber types31,32. While fibers of the same type are often adjacent in normal rat TA muscle, 97% of the time fibers were either isolated (85% of the fibers) or in “groups” of 2 (12% of the time; Fig. 4, open bar). We never observed more than 6 fibers adjacent to one another in normal muscle. In contrast, in any of the BoNT groups, isolated fibers or double fibers were only observed 74–85% of the time (Fig. 4, black, hatched, and grey bars). In other words, about 15% of the fibers in the BoNT groups appeared to be “grouped” compared to normal muscle fibers. While this may not have a functional effect, nearly 15% of the fibers being grouped implies that, following chemodenervation, sprouted axons innervate adjacent fibers and do not reinnervate the original neuromuscular junction. Therefore, the process of chemodenervation does not appear to be entirely reversible anatomically. This finding does not necessarily contradict the classic paper demonstrating reinnervation at the original neuromuscular junction,33 since our results do not eliminate the possibility of reinnervation at the original NMJ, but only demonstrate that it is not a universal phenomenon. Also, our method only investigated type 1 muscle fibers, which represent less than 0.5% of the fibers in rat TA muscle. The reinnervation status of the remaining 99.5% of type 2 fibers was not determined in this study. Interestingly, in spite of the increase in type 1 myosin heavy chain content measured in the injected muscles (Table 2), there was no parallel increase in the number of type 1 fibers measured in the muscle. These data are not necessarily contradictory. Since muscle fibers can express multiple myosin heavy chain isoforms, protein gel analysis of myosin content is not necessarily reflected in the immunohistochemical type of the fiber.
In summary, these experiments demonstrate that a second identical injection of BoNT 3 months after a previous injection causes a profound and persistent loss in muscle function and altered muscle structure. Structural abnormalities include fiber type transition, fiber type grouping, and lipid accumulation. The precise mechanisms for these changes are not clear.
Acknowledgments
Funding for this study was provided by the NIH (grants R24HD050837 and AR057013) and Allergan, Inc.
List of Abbreviations
- BoNT
onabotulinum toxin A (Botox®)
- NMJ
neuromuscular junction
- SEM
standard error of the mean
- TA
tibialis anterior muscle
Literature References
- 1.Brin MF. Botulinum toxin: chemistry, pharmacology, toxicity, and immunology. Muscle & nerve Supplement. 1997;6:S146–168. [PubMed] [Google Scholar]
- 2.Dolly O. Synaptic transmission: inhibition of neurotransmitter release by botulinum toxins. Headache. 2003;43(Suppl 1):S16–24. doi: 10.1046/j.1526-4610.43.7s.4.x. [DOI] [PubMed] [Google Scholar]
- 3.Meunier FA, Schiavo G, Molgo J. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. Journal of physiology, Paris. 2002;96:105–113. doi: 10.1016/s0928-4257(01)00086-9. [DOI] [PubMed] [Google Scholar]
- 4.Lowe K, Novak I, Cusick A. Repeat injection of botulinum toxin A is safe and effective for upper limb movement and function in children with cerebral palsy. Developmental medicine and child neurology. 2007;49:823–829. doi: 10.1111/j.1469-8749.2007.00823.x. [DOI] [PubMed] [Google Scholar]
- 5.Borodic GE, Ferrante R. Effects of repeated botulinum toxin injections on orbicularis oculi muscle. Journal of clinical neuro-ophthalmology. 1992;12:121–127. doi: 10.3109/01658109209058127. [DOI] [PubMed] [Google Scholar]
- 6.Elovic EP, Brashear A, Kaelin D, Liu J, Millis SR, Barron R, Turkel C. Repeated treatments with botulinum toxin type a produce sustained decreases in the limitations associated with focal upper-limb poststroke spasticity for caregivers and patients. Archives of physical medicine and rehabilitation. 2008;89:799–806. doi: 10.1016/j.apmr.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Herzog W, Longino D. The role of muscles in joint degeneration and osteoarthritis. Journal of biomechanics. 2007;40(Suppl 1):S54–63. doi: 10.1016/j.jbiomech.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Thomopoulos S, Kim HM, Rothermich SY, Biederstadt C, Das R, Galatz LM. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25:1154–1163. doi: 10.1002/jor.20418. [DOI] [PubMed] [Google Scholar]
- 9.Minamoto VB, Hulst JB, Lim M, Peace WJ, Bremner SN, Ward SR, Lieber RL. Increased efficacy and decreased systemic-effects of botulinum toxin A injection after active or passive muscle manipulation. Developmental medicine and child neurology. 2007;49:907–914. doi: 10.1111/j.1469-8749.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 10.Hulst JB, Minamoto VB, Lim MB, Bremner SN, Ward SR, Lieber RL. Systematic test of neurotoxin dose and volume on muscle function in a rat model. Muscle Nerve. 2014;49:709–715. doi: 10.1002/mus.23983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez-Castaneda J, Jankovic J. Long-term efficacy and safety of botulinum toxin injections in dystonia. Toxins. 2013;5:249–266. doi: 10.3390/toxins5020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters D, Barash IA, Burdi M, Yuan PS, Mathew L, Friden J, Lieber RL. Asynchronous functional, cellular and transcriptional changes after a bout of eccentric exercise in the rat. The Journal of physiology. 2003;553:947–957. doi: 10.1113/jphysiol.2003.048462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hentzen ER, Lahey M, Peters D, Mathew L, Barash IA, Friden J, Lieber RL. Stress-dependent and -independent expression of the myogenic regulatory factors and the MARP genes after eccentric contractions in rats. The Journal of physiology. 2006;570:157–167. doi: 10.1113/jphysiol.2005.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talmadge RJ, Roy RR, Edgerton VR. Muscle fiber types and function. Current opinion in rheumatology. 1993;5:695–705. doi: 10.1097/00002281-199305060-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lieber RL. The physiological basis of rehabilitation. Lippincott Williams & Wilkins; 2010. Skeletal muscle structure, function, and plasticity. [Google Scholar]
- 16.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiological reviews. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 17.Schroeder AS, Ertl-Wagner B, Britsch S, Schroder JM, Nikolin S, Weis J, Muller-Felber W, Koerte I, Stehr M, Berweck S, Borggraefe I, Heinen F. Muscle biopsy substantiates long-term MRI alterations one year after a single dose of botulinum toxin injected into the lateral gastrocnemius muscle of healthy volunteers. Movement disorders : official journal of the Movement Disorder Society. 2009;24:1494–1503. doi: 10.1002/mds.22661. [DOI] [PubMed] [Google Scholar]
- 18.Dulor JP, Cambon B, Vigneron P, Reyne Y, Nougues J, Casteilla L, Bacou F. Expression of specific white adipose tissue genes in denervation-induced skeletal muscle fatty degeneration. FEBS letters. 1998;439:89–92. doi: 10.1016/s0014-5793(98)01216-2. [DOI] [PubMed] [Google Scholar]
- 19.Wagatsuma A. Upregulation of gene encoding adipogenic transcriptional factors C/EBPalpha and PPARgamma2 in denervated muscle. Experimental physiology. 2006;91:747–753. doi: 10.1113/expphysiol.2006.033662. [DOI] [PubMed] [Google Scholar]
- 20.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation; research in biological diversity. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 21.Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature cell biology. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature cell biology. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 23.Eng CM, Smallwood LH, Rainiero MP, Lahey M, Ward SR, Lieber RL. Scaling of muscle architecture and fiber types in the rat hindlimb. The Journal of experimental biology. 2008;211:2336–2345. doi: 10.1242/jeb.017640. [DOI] [PubMed] [Google Scholar]
- 24.Gorin F, Herrick K, Froman B, Palmer W, Tait R, Carlsen R. Botulinum-induced muscle paralysis alters metabolic gene expression and fatigue recovery. The American journal of physiology. 1996;270:R238–245. doi: 10.1152/ajpregu.1996.270.1.R238. [DOI] [PubMed] [Google Scholar]
- 25.Moreau N, Li L, Damiano DL. A feasible and reliable muscle fatigue assessment protocol for individuals with cerebral palsy. Pediatric physical therapy : the official publication of the Section on Pediatrics of the American Physical Therapy Association. 2008;20:59–65. doi: 10.1097/PEP.0b013e31815e410c. [DOI] [PubMed] [Google Scholar]
- 26.Moreau NG, Li L, Geaghan JP, Damiano DL. Contributors to fatigue resistance of the hamstrings and quadriceps in cerebral palsy. Clinical biomechanics. 2009;24:355–360. doi: 10.1016/j.clinbiomech.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microscopy research and technique. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Dodd SL, Selsby J, Payne A, Judge A, Dott C. Botulinum neurotoxin type A causes shifts in myosin heavy chain composition in muscle. Toxicon : official journal of the International Society on Toxinology. 2005;46:196–203. doi: 10.1016/j.toxicon.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Inagi K, Connor NP, Schultz E, Ford CN, Cook CH, Heisey DM. Muscle fiber-type changes induced by botulinum toxin injection in the rat larynx. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1999;120:876–883. doi: 10.1016/S0194-5998(99)70330-X. [DOI] [PubMed] [Google Scholar]
- 30.Kranjc BS, Sketelj J, D’Albis A, Erzen I. Long-term changes in myosin heavy chain composition after botulinum toxin a injection into rat medial rectus muscle. Investigative ophthalmology & visual science. 2001;42:3158–3164. [PubMed] [Google Scholar]
- 31.Engel AG, Banker B. Myology. McGraw-Hill; 1986. [Google Scholar]
- 32.Carpenter S, Karpati G. Pathology of skeletal muscle. New York: Churchill Livingstone; 1984. [Google Scholar]
- 33.de Paiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3200–3205. doi: 10.1073/pnas.96.6.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]