SUMMARY
SETTING
Active case finding is a World Health Organization (WHO) endorsed strategy for improving tuberculosis (TB) case detection. Despite WHO recommendations for active case finding among people who inject drugs (PWID), few studies have been published. The historical focus of case finding has been in populations that are human immunodeficiency virus-positive, incarcerated or at higher occupational risk.
OBJECTIVE
We sought to examine the yield of active case finding among PWID newly started on methadone in Tanzania.
DESIGN
Of 222 methadone clients, 156 (70%) met with study administrators; 150 consented to participate, 139 (93%) of whom were male. The median age was 34 years. A symptom-based questionnaire was developed by the investigators and administered to every consenting patient by a native Swahili speaker.
RESULTS
Of the 150 patients surveyed, 16 (11%) had one or more TB symptoms and were referred for laboratory testing. Six new TB cases were identified in this active case finding program, with a prevalence of 4%.
CONCLUSION
This study presents the first data on TB prevalence in a population of PWID in Tanzania. This prevalence is 23 times that of the general Tanzanian TB prevalence of 0.2%. These results have significant implications for TB control.
Keywords: tuberculosis, methadone, injection drug use, Tanzania
EAST AFRICA has been an important stop along international drug trafficking routes for three decades.1 The United Nations Office of Drugs and Crime estimated that there were 1 736 000 heroin users in Africa in 2011, of whom approximately a third reside in Eastern Africa.1 As of 2005, there were an estimated 200 000–250 000 individuals using heroin in Tanzania, with approximately 40 000 injectors.2 Although methadone treatment is available, demand for treatment outpaces supply.
The epidemics of tuberculosis (TB) and the human immunodeficiency virus (HIV) are intertwined with heroin addiction in Tanzania. An estimated 42% of people who inject drugs (PWID) in Dar es Salaam have HIV.3 These HIV-infected injectors have a more than 20- to 37-fold risk of TB compared to non-HIV-infected persons.4,5 As of 2009, Tanzania ranked fifteenth among the 22 high TB burden countries.6 In 2011, the World Health Organization (WHO) estimated TB prevalence and incidence in Tanzania to be respectively 0.2% and 0.2%, with 1.1% multidrug-resistant TB (MDR-TB) cases.7 The WHO recommends that all services dealing with drug users have a case-finding protocol; at minimum, it recommends administering a symptom questionnaire to screen for active TB.8
Methadone was introduced in 2011 at the Muhimbili National Hospital in Dar es Salaam, Tanzania, to address heroin addiction with the goal of reducing injection use, preventing HIV and helping those with HIV to engage in care and treatment. This was the first public methadone clinic in sub-Saharan Africa, and all clients were heroin injectors. An early methadone patient died of TB, prompting concerns that others may have TB. These undiagnosed cases could be lethal without early treatment, and could pose an infection risk to fellow patients and health care workers. In response to this, and in line with WHO recommendations for screening drug users, we implemented an active case-finding program among methadone clients to assess prevalence and link cases to treatment.
METHODS
The study was conducted at the Medically Assisted Treatment (MAT) program in the Psychiatry Department at Muhimbili National Hospital, Dar es Salaam. Our study population included all 222 registered patients who were regularly attending the MAT program as of 22 August 2011, when enrollment concluded.
An informational flyer was posted outside the methadone-dispensing window several days in advance of the study to inform patients of the upcoming screening. The pharmacist directed patients to meet with study administrators onsite after receiving methadone. All participants provided informed consent before screening.
A symptom-based questionnaire was developed by the investigators and administered to every consenting patient by a native Swahili speaker. Patients were asked the following questions: cough >2 weeks' duration (with or without sputum, with or without hemoptysis), fever >1 month's duration, night sweats, weight loss and change in appetite. If the patient reported a cough of <2 weeks' duration, he/she was asked to return at 2 weeks' duration to report if the cough had persisted. In addition, patients were asked about past TB history, housing and ventilation, and history of incarceration (including pre-trial detention).
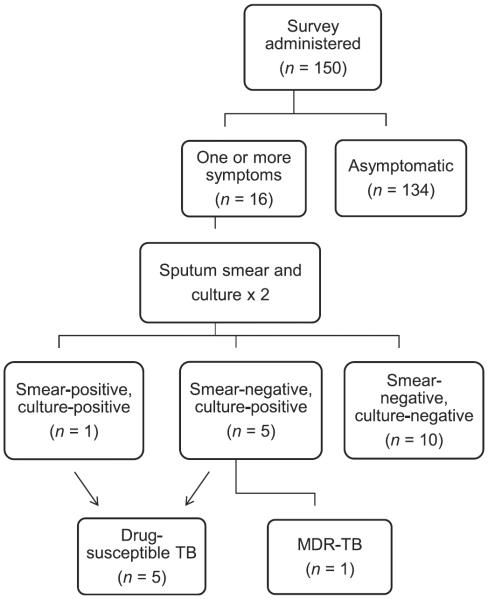
Any patient reporting one or more symptoms in the questionnaire was referred for sputum collection (Figure). Patients were asked to produce two sputum samples: one onsite after completion of the questionnaire and without exposure to direct sunlight, and the other the following morning and delivered to clinic staff when the patient attended for methadone. Samples were stored indoors in a cool environment, out of direct sunlight, for no longer than 12 h. The study administrator delivered the samples, again without exposure to sunlight, to the Central Tuberculosis Reference Laboratory located onsite at Muhimbili National Hospital.
Figure.
Flow chart of survey and laboratory testing protocol. TB = tuberculosis; MDR-TB = multidrug-resistant TB.
Sputum smear analysis was performed by a trained technician using auramine-O staining. Each sample was cultured using Löwenstein-Jensen solid media (modified Petroff method). Culture-positive samples underwent drug susceptibility testing (DST) against isoniazid, rifampin, streptomycin and ethambutol. Samples with suspected mycobacteria other than tuberculosis (MOTT) were confirmed using ρ-nitro-benzoic acid (PNB).
The MAT program was informed of all patients with smear or culture results positive for TB, and these patients were referred to the National Tuberculosis and Leprosy Programme (NTLP) treatment site based at Muhimbili National Hospital. Treatment was initiated by the NTLP according to national guidelines.
As part of the MAT clinical program, patients undergo standardized assessments, including the addiction severity index (ASI), throughout the course of their treatment. The ASI is a standardized instrument used to establish the nature and severity of possible medical, employment, drug, alcohol, legal, family, social and psychiatric problems. A chart review was performed for information from the ASI and for HIV status. HIV positivity was defined by any previous HIV-positive result, whether at the MAT clinic or at another facility. HIV-negative status was defined by a negative test at the MAT clinic within the previous 3 months. All other patients were classified as unknown HIV status. For patients not enrolled in our study, we collected data from baseline registration information and laboratory tests, and compared them with our sample using two-sample Student's t-test for means and two-sample test for proportions. Data were analyzed using Microsoft Excel (Microsoft, Redmond, WA, USA) and Stata version 2.0 (Stata Corp, College Station, TX, USA).
This study was cleared by two Institutional Review Boards: the Yale University Human Investigations Committee, New Haven, CT, USA, granted an exemption from review, and the Muhimbili University of Health and Allied Sciences Senate Research & Publications Committee, Dar es Salaam, Tanzania, granted ethical approval.
RESULTS
From 28 July to 22 August 2011, 156 of 222 (70%) clients met with study administrators, of whom 150 consented to participate in this study. Table 1 compares age, sex, HIV positivity rate and previous history of arrest or charge between our sample and the 72 patients not enrolled in the study. Our sample was older than the unenrolled group, and had a significantly higher percentage of known HIV-positives.
Table 1.
Baseline characteristics for all patients on methadone treatment as of 22 August 2011 at the Muhimbili Medication Assisted Treatment Program, Dar es Salaam, Tanzania*
| All registered patients (n =222) | Enrolled patients (n = 150) | Non-enrolled patients (n = 72) | ||
|---|---|---|---|---|
| Characteristics | n (%) | n (%) | n (%) | P value |
| Age, years, mean ± SD | 33.3 ± 5.7 | 34.1 ± 5.9 | 31.5 ± 5.1 | 0.002 |
| Male | 204 (92) | 139 (93) | 65 (90) | 0.54 |
| HIV-positive | 42 (19) | 34 (23) | 8 (11) | 0.03 |
| Previous arrest or charge | 138 (62) | 88 (59) | 50 (69) | 0.12 |
All information taken from chart review.
SD = standard deviation; HIV = human immunodeficiency virus.
Demographics, risk factors and TB history of our sample are given in Table 2. The median age was 34 years (interquartile range [IQR] 8). Men comprised the largest group, with 139/150 (93%) participants. All patients had a previous history of heroin injection, as this was required for entry into the methadone program. In addition to heroin, marijuana (36%) and alcohol (11%) were the two most common substances used. The majority of patients (69%) reported living with others, with a mean of five members per household. A history of incarceration, including prison, jail or pre-trial detention, was reported by 107 (71%) patients. Chart review showed that 34 (23%) were HIV-positive and 12 (8%) patients had tested negative for HIV in the last 3 months. Despite the elevated HIV risk among PWID, 104 (69%) refused HIV testing, and their status was unknown. A previous history of TB was reported by 26 patients (17%); the median time since previous diagnosis was 5 years (IQR 5.5). No laboratory data were available to confirm prior TB. In their previous diagnosis of TB, 14 (54%) patients reported diagnosis by laboratory methods. Twenty-four (92%) patients completed a full course of treatment, with 10 (38%) completing an 8-month course and 14 (54%) completing a 6-month course. Only two patients (8%) reported a history of defaulting from anti-tuberculosis treatment.
Table 2.
Population demography, tuberculosis risk factors, HIV status and TB history of patients on methadone treatment at the Muhimbili Medically Assisted Treatment Program, Dar es Salaam, Tanzania
| Total (n = 150) | Non-TB patients (n = 144) | TB patients (n = 6) | |
|---|---|---|---|
| Characteristics | n/N (%) | n/N (%) | n/N (%) |
| Age, years, median [IQR] | 34 [8] | 34 [8] | 34.5 [6.25] |
| Sex | |||
| Male | 139/150 (93) | 133/144 (92) | 6/6 (100) |
| Female | 11/150 (7) | 11/144 (8) | 0 |
| Marital status | |||
| Single | 92/150 (61) | 89/144 (62) | 3/6 (50) |
| Married | 25/150 (17) | 23/144 (16) | 2/6 (33) |
| Divorced/separated | 30/150 (20) | 29/144 (20) | 1/6 (17) |
| Widowed | 3/150 (2) | 3/144 (2) | 0 |
| Children, mean n ± SD | 1.0 ± 0.9 | 1.0 ± 0.9 | 1.0 ± 0.9 |
| Education | |||
| No education | 1/150 (0.7) | 1/144 (0.7) | 0 |
| Primary | 70/150 (47) | 66/144 (46) | 4/6 (67) |
| Low secondary | 62/150 (41) | 60/144 (42) | 2/6 (33) |
| Upper secondary | 11/150 (7) | 11/144 (8) | 0 |
| University | 6/150 (4) | 6/144 (4) | 0 |
| Additional training* | 13/150 (9) | 13/144 (9) | 0 |
| Primary substance of abuse (other than heroin) | |||
| None | 70/150 (47) | 68/144 (47) | 2/6 (33) |
| Alcohol | 17/150 (11) | 15/144 (10) | 2/6 (33) |
| Sedatives, hypnotics, tranquilizers | 6/150 (4) | 6/144 (4) | 0 |
| Cannabis | 54/150 (36) | 52/144 (36) | 2/6 (33) |
| Polysubstance | 3/150 (2) | 3/144 (2) | 0 |
| Housing | |||
| Own | 16/150 (11) | 15/144 (10) | 1/6 (17) |
| Rent | 29/150 (19) | 29/144 (20) | 0 |
| Rent, TSH, mean ± SD | 37 600 ± 25 700 | 37 600 ± 25 700 | NA |
| Living with others | 103/150 (69) | 98/144 (68) | 5/6 (83) |
| Homeless | 2/150 (1) | 2/144 (1) | 0 |
| Years in same house, mean ± SD | 12.0 ± 14.1 (148 entries) | 12.0 ± 14.2 (141 entries) | 11.4 ± 13.1 |
| Number of household members, mean ± SD | 4.9 ± 2.6 (145 entries) | 4.9 ± 2.6 (139 entries) | 4.6 ± 2.7 (7 entries) |
| Number of windows per room, mean ± SD | 1.5 ± 0.6 (148 entries) | 1.6 ± 0.6 (142 entries) | 1.16 ± 0.4 |
| Previously incarcerated or in jail† | 107/150 (71) | 102/144 (71) | 5/6 (83) |
| Knowledge about TB (1–5), mean ± SD‡ | 2.4 ± 1.0 | 2.4 ± 1.0 | 2.0 ± 0.6 |
| Risk of TB (1–5), mean ± SD‡ | 1.8 ± 1.0 | 1.8 ± 1.0 (n = 143) | 3.5 ± 0.5 |
| Effort to prevent TB (1–5), mean ± SD‡ | 3.5 ± 1.2 | 3.5 ± 1.2 | 2.3 ± 0.5 |
| HIV status | |||
| HIV-positive | 34/150 (23) | 31/144 (22) | 3/6 (50) |
| HIV-negative | 12/150 (8) | 11/144 (8) | 1/6 (17) |
| Unknown HIV status | 104/150 (69) | 102/144 (71) | 2/6 (33) |
| Previous history of TB | 26/150 (17) | 23/144 (16) | 3/6 (50) |
| Years since last diagnosis median, [IQR] | 5.0 [5.5] | 5 [6.5] | 4.5 [2] |
| Type of TB, most recent | |||
| Pulmonary | 19/26 (73) | 17/23 (74) | 2/3 (67) |
| Extra-pulmonary | 6/26 (23) | 6/23 (26) | 1/3 (33) |
| Not sure | 1/26 (4) | 1/23 (4) | 0 |
| How diagnosed, most recent | |||
| Laboratory methods | 14/26 (54) | 12/23 (52) | 2/3 (67) |
| X-ray | 9/26 (35) | 8/23 (35) | 1/3 (33) |
| Laboratory and X-ray | 3/26 (12) | 3/23 (13) | 0 |
| Amount of treatment, most recent | |||
| Complete, 8 months | 10/26 (38) | 10/23 (43) | 1/3 (33) |
| Complete, 6 months | 14/26 (54) | 13/23 (57) | 1/3 (33) |
| Incomplete, 5–6 months | 1/26 (4) | 0/23 (0) | 1/3 (33) |
| Incomplete, <2 months | 1/26 (4) | 1/23 (4) | 0 |
| Total completing treatment | 24/26 (92) | 22/23 (96) | 2/3 (67) |
| Total defaulting from treatment | 2/26 (8) | 1/23 (4) | 1/3 (33) |
| Most recent test after treatment | |||
| Positive | 0 | 0/23 (0) | 0 |
| Negative | 21/26 (81) | 19/23 (83) | 2/3 (67) |
| Not tested after beginning treatment | 5/26 (19) | 4/23 (17) | 1/3 (33) |
All of these belonged to one of the categories above as well as additional training.
These data come from the administered questionnaire, and are not equivalent to `previous arrest or charge' in Table 1, which was taken from the baseline addiction severity index.
Patients were asked to rate their knowledge of TB, risk of TB and efforts to prevent TB on a scale of 1–5.
HIV = human immunodeficiency virus; TB = tuberculosis; IQR = interquartile range; SD = standard deviation; TSH = Tanzanian shillings.
Of the 150 patients surveyed, 16 (11%) with one or more symptoms were referred for laboratory testing (Table 3). The sputum sample from one patient was acid-fast bacilli positive on sputum smear, and subsequently culture-positive. Culture of sputum samples from the remaining 15 smear-negative patients grew Mycobacterium tuberculosis for 5 patients; 1 patient was found to have MOTT. On DST of culture-positive cases, one sputum smear-negative patient was found to have MDR-TB. The sputum sample for one smear-negative, culture-positive patient was contaminated during DST. All patients identified with TB had a productive cough of >2 weeks' duration. Of the 13 patients reporting cough, six (46%) had TB. All patients who reported hemoptysis were found to have TB.
Table 3.
TB laboratory testing results of patients on methadone treatment at the Muhimbili Medically Assisted Treatment Program, Dar es Salaam, Tanzania
| Characteristic | n/N (%) |
|---|---|
| Total patients surveyed | 150 |
| Symptom-positive | 16/150 (11) |
| Smear-positive, culture-positive | 1/16 (6) |
| Smear-negative, culture-positive* | 5/16 (31) |
| Smear-negative, culture-negative | 10/16 (63) |
| New TB cases | 6/150 (4) |
| Multidrug-resistant TB† | 1/6 (17) |
On sputum culture, one patient with unknown HIV status was found to have mycobacteria other than TB. This patient was not considered a positive case, but may have had an opportunistic infection.
One sputum sample was contaminated during drug susceptibility testing.
TB = tuberculosis.
Overall, six new TB cases were identified in this active case-finding program, with a prevalence of 4% (Table 3). The demographics of those with and those without TB are presented in Table 2. Of note, 5/6 TB patients (83%) lived with on average 4.6 other individuals, and 5/6 patients (83%) had a history of incarceration. Three of the TB patients were HIV-infected, two were HIV-negative, and the remaining patient had unknown HIV status. Three of the patients reported a history of TB; two of these (67%) reported completing their previous course of treatment, while one had defaulted from treatment.
DISCUSSION
In our sample of 150 PWID on methadone maintenance, 4% had active pulmonary TB. This is roughly 23 times the national Tanzanian TB prevalence of 0.2% in 2011.7 To our knowledge, this study presents the first data on TB in a population of PWID in Tanzania. Other Tanzanian studies on active TB have been performed in different populations with varying rates of TB.9–15 Among HIV voluntary counseling centers in Dar es Salaam, for example, 35/1280 patients (2.7%) were culture-positive.14 Case-finding studies for TB in methadone clinics have not previously been performed in Africa; however, they have been performed elsewhere.16–20 Selwyn et al. examined rates of TB activation among HIV-infected drug users on methadone in New York City, NY, USA, and found that 4% of individuals who were purified protein derivative positive later developed active TB.17 Another study that screened 112 methadone patients in Estonia found no active cases of TB.20
Our results have significant implications for TB control in this population. Tanzanian PWID are a subpopulation at high risk for TB, and should be actively screened. If passive case finding alone had continued, these individuals would have remained undiagnosed for a longer period of time, with potentially lethal consequences. As methadone patients are required to attend the clinic 7 days a week, these undiagnosed cases also presented a transmission risk for other MAT clients and their health care providers. The original layout of the clinic's waiting area led to crowded conditions that allowed for ample opportunity for airborne transmission. The high proportion of patients with active pulmonary TB prompted the clinic staff to modify the structure of the waiting room to increase the number of air changes per hour. The program was thus beneficial for both cases and their regular contacts, including patients and staff.
As five of the six patients with active TB in our sample lived with others, household contacts were at increased risk of infection. A 2009 study from Dar es Salaam found Mantoux skin test reactivity in more than 60% of household contacts of smear-positive cases.21 The detection of at least one MDR-TB case makes the potential for transmission beyond the PWID community even more worrisome.
In our study, five (83%) of the six newly diagnosed TB cases were missed by sputum smear and identified only on culture. One potential explanation for this finding is that HIV-infected individuals are more likely to have sputum-smear negative TB.22 Two studies among HIV-infected patients in Tanzania have shown smear sensitivity of 40%15 and 61.8%.12 Of our 5 smear-negative, culture-positive patients, 2 were known to be HIV-infected, and 2 had unknown HIV status. Our results suggest that sputum culture should be included in all active screening programs of PWID. Of critical importance, the majority of our patients were diagnosed only by sputum culture, which took up to several weeks to obtain a result. These patients continued to pose an infection risk during this diagnostic delay, highlighting the need for rapid diagnostics in screening programs.
Our symptom analysis showed that cough >2 weeks and sputum production were the most sensitive symptoms, with all new diagnoses having these symptoms. This is consistent with other studies, which have successfully used cough of >2–3 weeks as the only symptom screen.23 In one Tanzanian study of HIV-infected patients with cough, 9/125 (7.2%) had smear-positive TB.9 This is similar to the 1/13 (7.7%) patients with cough in our study, although 6/13 (46%) were culture-positive.
An analysis of population characteristics reveals several interesting facts. First, there was a very high rate of past TB history in the population as a whole (17%), which was even higher among patients currently infected with TB (50%). One study of HIV patients in Dar es Salaam found that 13.8% of 80 subjects with a previous history of TB developed recurrent TB within a mean follow-up period of 3.2 years,11 which is similar to our rate of 11.5% (3/26). Furthermore, a very high rate of patients had been previously incarcerated or held in pre-trial detention (71%), which may have served as a major transmission site for PWID.
Our study has several limitations. First, our study used a small, non-random sample, and was susceptible to selection bias. Our sample had a significantly higher known HIV positivity rate than unenrolled patients. There are two potential explanations. First, chart review was more thorough for patients in the sample, taking into account patient's self-report of positive status, while chart review for unenrolled patients was limited to registration data and laboratory testing. Second, patients who refused or avoided recruitment into our study may also have been more likely to refuse HIV testing, and may have had undiagnosed HIV infection. Another limitation was that only symptomatic patients underwent laboratory testing (with an emphasis on pulmonary symptoms), thus missing asymptomatic cases and those with extra-pulmonary TB. Asymptomatic cases should also be considered, as found in a study of 93 HIV patients in Dar es Salaam, where four asymptomatic cases were detected.13 In a rural Tanzanian HIV clinic study, 75% of the 20 cases diagnosed were asymptomatic.15 Without data on asymptomatic cases, we could not calculate the true sensitivity and specificity of our screening questions. Third, as 69% of the sample had unknown HIV status, this limited our ability to analyze rates of HIV-TB co-infection in this population. Fourth, the majority of our patients were male, reflecting the sex distribution of the clinic as a whole. Our data thus provide limited insight into TB among female injectors. Finally, we defined a TB case as smear- or culture-positive sputum—this may underestimate the TB prevalence in the MAT program in comparison with national prevalence rates that include cases that are not microbiologically confirmed.
The clients participating in the MAT program were selected based on their motivation and likelihood to succeed in methadone treatment; it is therefore reasonable to believe that they represent a healthier subset of the larger PWID population, who may have a higher burden of HIV and TB. Future efforts should aim to expand active case finding to the larger community of PWID in Tanzania, as well as their household contacts.
CONCLUSION
This pilot study actively screened 150 PWID who were started on methadone in Dar es Salaam, Tanzania, and found that six patients had sputum cultures indicative of active TB. This TB prevalence of 4% is 23 times higher than the national prevalence of TB in Tanzania. Our study suggests that sputum culture and DST should be obtained on all patients with a productive cough of >2 weeks. Ongoing efforts to identify PWID with TB must be undertaken, and effective treatment programs must be established to assist patients in completing treatment.
Acknowledgements
The authors wish to thank O Chang for help with data analysis.
Footnotes
Conflict of interest: none declared.
References
- 1.United Nations Office on Drugs and Crime . The global Afghan opium trade: a threat assessment. UNODC; Vienna, Austria: [Accessed April 2014]. 2011. http://www.unodc.org/documents/data-and-analysis/Studies/Global_Afghan_Opium_Trade_2011-web.pdf. [Google Scholar]
- 2.McCurdy SA, Williams ML, Kilonzo GP, Ross MW, Leshabari MT. Heroin and HIV risk in Dar es Salaam, Tanzania: youth hangouts, mageto and injecting practices. AIDS Care. 2005;17(Suppl 1):S65–S76. doi: 10.1080/09540120500120930. [DOI] [PubMed] [Google Scholar]
- 3.Williams ML, McCurdy SA, Bowen AM, et al. HIV seroprevalence in a sample of Tanzanian intravenous drug users. AIDS Educ Prev. 2009;21:474–483. doi: 10.1521/aeap.2009.21.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Getahun H, Gunneberg C, Sculier D, Verster A, Raviglione M. Tuberculosis and HIV in people who inject drugs: evidence for action for tuberculosis, HIV, prison and harm reduction services. Curr Opin HIV AIDS. 2012;7:345–353. doi: 10.1097/COH.0b013e328354bd44. [DOI] [PubMed] [Google Scholar]
- 5.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 6.Joint United Nations Programme on HIV/AIDS . United Republic of Tanzania HIV and AIDS estimates. UNAIDS; Geneva, Switzerland: 2009. [Google Scholar]
- 7.World Health Organization . WHO/HTM/TB/2012.6. WHO; Geneva, Switzerland: 2012. Global tuberculosis report, 2012. [Google Scholar]
- 8.World Health Organization . WHO/HTM/TB/2008.404. WHO/HIV/2008.750. WHO; Geneva, Switzerland: [Accessed April 2014]. 2008. Policy guidelines for collaborative TB and HIV services for injecting and other drug users: an integrated approach. http://whqlibdoc.who.int/publications/2008/9789241596930_eng.pdf. [PubMed] [Google Scholar]
- 9.Mwita J, Mugusi F, Pallangyo K. Pneumocyctis pneumonia and pulmonary tuberculosis among HIV-infected patients at Muhimbili National Hospital, Tanzania. East Afr J Public Health. 2012;9:10–12. [PubMed] [Google Scholar]
- 10.Peck RN, Luhanga A, Kalluvya S, et al. Predictors of tuberculosis in first 6 months after initiation of antiretroviral therapy: a case-control study. Int J Tuberc Lung Dis. 2012;16:1047–1051. doi: 10.5588/ijtld.11.0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lahey T, Mackenzie T, Arbeit RD, et al. Recurrent tuberculosis risk among HIV-infected adults in Tanzania with prior active tuberculosis. Clin Infect Dis. 2013;56:151–158. doi: 10.1093/cid/cis798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matee M, Mtei L, Lounasvaara T, et al. Sputum microscopy for the diagnosis of HIV-associated pulmonary tuberculosis in Tanzania. BMC Public Health. 2008;8:68. doi: 10.1186/1471-2458-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40:1500–1507. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 14.Munseri PJ, Bakari M, Pallangyo K, Sandstrom E. Tuberculosis in HIV voluntary counseling and testing centres in Dar es salaam, Tanzania. Scand J Infect Dis. 2010;42:767–774. doi: 10.3109/00365548.2010.495725. [DOI] [PubMed] [Google Scholar]
- 15.Ngowi BJ, Mfinanga SG, Bruun JN, Morkve O. Pulmonary tuberculosis among people living with HIV/AIDS attending care and treatment in rural northern Tanzania. BMC Public Health. 2008;8:341. doi: 10.1186/1471-2458-8-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichman LB, Felton CP, Edsall JR. Drug dependence, a possible new risk factor for tuberculosis disease. Arch Intern Med. 1979;139:337–339. [PubMed] [Google Scholar]
- 17.Selwyn PA, Hartel D, Lewis VA, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 18.Daley CL, Hahn JA, Moss AR, Hopewell PC, Schecter GF. Incidence of tuberculosis in injection drug users in San Francisco: impact of anergy. Am J Respir Crit Care Med. 1998;157:19–22. doi: 10.1164/ajrccm.157.1.9701111. [DOI] [PubMed] [Google Scholar]
- 19.Conover C, Ridzon R, Valway S, et al. Outbreak of multidrug-resistant tuberculosis at a methadone treatment program. Int J Tuberc Lung Dis. 2001;5:59–64. [PubMed] [Google Scholar]
- 20.Ruutel K, Loit HM, Sepp T, Kliiman K, McNutt LA, Uuskula A. Enhanced tuberculosis case detection among substitution treatment patients: a randomized controlled trial. BMC Res Notes. 2011;4:192. doi: 10.1186/1756-0500-4-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kifai EJ, Bakari M. Mantoux skin test reactivity among household contacts of HIV-infected and HIV un-infected patients with sputum smear positive TB in Dar es Salaam, Tanzania. East Afr J Public Health. 2009;6:211–218. doi: 10.4314/eajph.v6i2.51786. [DOI] [PubMed] [Google Scholar]
- 22.Kranzer K, Houben RM, Glynn JR, Bekker LG, Wood R, Lawn SD. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:93–102. doi: 10.1016/S1473-3099(09)70326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corbett EL, Charalambous S, Moloi VM, et al. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med. 2004;170:673–679. doi: 10.1164/rccm.200405-590OC. [DOI] [PubMed] [Google Scholar]