Abstract
Background and Purpose
Rapid ventricular pacing (RVP) applied before an index ischaemia has anti-ischaemic effects. Here, we investigated whether RVP applied after index ischaemia attenuates reperfusion injury and whether peroxynitrite, reperfusion injury salvage kinase (RISK) and survival activating factor enhancement (SAFE) pathways as well as haem oxygenase 1 (HO1) are involved in the mechanism of RVP-induced postconditioning.
Experimental Approach
Langendorff perfused rat hearts were subjected to 30 min regional ischaemia and 120 min reperfusion with or without ischaemic postconditioning (6 × 10/10 s reperfusion/ischaemia; IPost) or RVP (6 × 10/10 s non-pacing/rapid pacing at 600 bpm) applied at the onset of reperfusion.
Key Results
Meta-analysis of our previous studies revealed an association between longer reperfusion-induced ventricular tachycardia/fibrillation with decreased infarct size. In the present experiments, we tested whether RVP is cardioprotective and found that both IPost and RVP significantly decreased infarct size; however, only RVP attenuated the incidence of reperfusion-induced ventricular tachycardia. Both postconditioning methods increased the formation of cardiac 3-nitrotyrosine and superoxide, and non-significantly enhanced Akt phosphorylation at the beginning of reperfusion without affecting ERK1/2 and STAT3, while IPost alone induced HO1. Application of brief ischaemia/reperfusion cycles or RVP without preceding index ischaemia also facilitated peroxynitrite formation; nevertheless, only brief RVP increased STAT3 phosphorylation.
Conclusions and Implications
Short periods of RVP at the onset of reperfusion are cardioprotective and increase peroxynitrite formation similarly to IPost and thus may serve as an alternative postconditioning method. However, downstream mechanisms of the protection elicited by IPost and RVP seem to be partially different.
Linked Articles
This article is part of a themed section on Conditioning the Heart – Pathways to Translation. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-8
Tables of Links
| TARGETS | |||
|---|---|---|---|
| Acetylcholinesterase | ERK1 | HO1 | PKG |
| Akt (PKB) | ERK2 | PKC |
| LIGANDS | |
|---|---|
| cGMP | Nitric oxide (NO) |
| CGRP |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Ischaemic heart diseases, including acute myocardial infarction, are the leading cause of death in industrialized countries. Reperfusion therapy for infarction allows rapid return of blood flow to the ischaemic myocardium and decreases mortality rate. However, early reperfusion itself is accompanied by deleterious events: the occurrence of life-threatening arrhythmias, no-reflow phenomenon, myocardial stunning and additional cell death (Yellon and Hausenloy, 2007). This paradoxical reperfusion injury caused by the restoration of blood flow and oxygen supply (Yamada et al., 1990) leads to increased infarct size, impaired contractile function and electrical vulnerability, largely compromising clinical outcomes.
Ischaemic postconditioning (IPost) has emerged in the last decade as a potential therapeutic intervention for limiting reperfusion injury (Zhao et al., 2003; Ovize et al., 2010). The procedure is based upon the application of brief cycles of ischaemia/reperfusion (I/R) immediately after a prolonged ischaemia and it has been reported to reduce myocardial damage both in animal studies and in human clinical trials (Ovize et al., 2010). Nevertheless, some studies have reported the ineffectiveness of IPost both in animals and in humans (Dow and Kloner, 2007; Hahn et al., 2013). A possible explanation for the controversial results could be that the outcome of postconditioning may depend on several factors, such as failure to achieve complete reperfusion during application of brief I/R cycles, the duration of index ischaemia, the algorithm of postconditioning manoeuvre, gender, age and temperature (Skyschally et al., 2009b). In addition, co-morbidities, such as hyperlipidaemia (Kupai et al., 2009) and diabetes (Miki et al., 2012), may interfere with the infarct size-limiting effect of postconditioning. These confounding factors indicate the necessity to develop new alternative methods and models to induce postconditioning.
Heart rate is known to play a role in the development of I/R injury (Bernier et al., 1989), and it was shown that either slowing or increasing heart rate before ischaemia limits myocardial injury (Tosaki et al., 1988; Bernier et al., 1989; Hearse et al., 1999). Moreover, we have previously shown that short periods of rapid ventricular pacing (RVP) applied before an index ischaemia has anti-ischaemic effects (pacing-induced preconditioning) (Ferdinandy et al., 1997a, b; 1998). However, the effect of short periods of RVP performed at the early phase of reperfusion has not been investigated so far.
The exact molecular mechanism of myocardial postconditioning is not entirely clear. Increasing evidence suggests that enhanced formation of cardiac peroxynitrite is involved in cardioprotection afforded by both pre- (Altug et al., 2000; Altup et al., 2001; Csonka et al., 2001) and postconditioning (Kupai et al., 2009; Li et al., 2013). Kupai et al. reported first that IPost failed to decrease infarct size in the presence of a peroxynitrite decomposition catalyst, thereby suggesting essential triggering role of peroxynitrite in postconditioning-induced cardioprotection (Kupai et al., 2009).
Therefore, here we aimed to investigate whether RVP applied after index ischaemia has any effect on the markers of reperfusion injury and we studied the role of peroxynitrite in the mechanisms of postconditioning. Furthermore, we looked at activation of reperfusion injury salvage kinase (RISK) and survival activating factor enhancement (SAFE) pathways and haem oxygenase 1 (HO1) as possible downstream targets of RVP-induced postconditioning.
Methods
Male Wistar rats were used in our previous and present (n = 74) studies. The studies conform to the ‘Guide for the care and use of laboratory animals’ published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and was approved by local ethics committees. The animals were kept at 12/12h light/dark cycle and had free access to standard laboratory chow and drinking water. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Isolated heart preparation
Isolated heart preparation was carried out as described in our previous studies with slight modifications (Ferdinandy et al., 1997a; Kocsis et al., 2012; Varga et al., 2014). Inhalation anaesthesia of rats was induced in a glass desiccator containing cellulose wadding soaked in diethyl ether, an anaesthetic not known to interfere with cardioprotection. During isolation of the heart, rats were removed from the chamber and a beaker containing wadding soaked in ether was held near the muzzle of rats in order to maintain anaesthesia. Rats were given 500 U·kg−1 heparin i.v. Hearts were then isolated and perfused according to Langendorff at 37°C with Krebs–Henseleit buffer containing 118 mM NaCl, 25 mM NaHCO3, 4.3 mM KCl, 1.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 11 mM glucose, gassed with 95% O2 and 5% CO2. Hydrostatic perfusion pressure was kept constant at 100 cmH2O (9.8 kPa) throughout the experiments. Coronary flow was measured by collecting coronary effluent for a period of time and was expressed as mL·min−1.
A 3-0 silk suture was placed around the left anterior descending coronary artery (LAD) close to its origin and the snare was tightened by applying a 100 g hanging weight to induce regional index ischaemia. For IPost, brief no-flow global ischaemia was performed by turning off the perfusion cannula. The presence of ischaemia was verified by monitoring coronary flow. RVP (600 bpm; 10 Hz) was performed by an electric stimulator (Experimetria Inc., Budapest, Hungary) with double threshold square, 1 V, 1 mA and 5 ms impulses conducted by electrodes attached directly to the surface of the right ventricle close to the apex and to the aortic cannula as described previously (Ferdinandy et al., 1997a, b; 1998). Heart rates were monitored (Isosys; Experimetria Inc.) by recording epicardial ECG throughout the whole duration of perfusion.
Relationship between the duration of reperfusion-induced ventricular tachyarrhythmia and infarct size: a meta-analysis
Meta-analysis was performed on ECGs and infarct size data from our six previous studies performed in our laboratory on isolated rat hearts subjected to 30 min regional ischaemia and 120 min reperfusion (Figure 1A). Reperfusion-induced arrhythmias were analysed in the first 10 min of reperfusion. Hearts presenting sustained (>10 min) tachyarrhythmia were excluded (n = 14). Three separate evaluations were performed based on the total duration of ventricular tachycardia (VT), ventricular fibrillation (VF) or VT + VF respectively. Infarct size data were presented on the basis of duration (shorter or longer than 60 s) of VT, VF or VT + VF. Infarct size data exceeding mean ± 2 SD were excluded from the analysis (n = 6).
Figure 1.
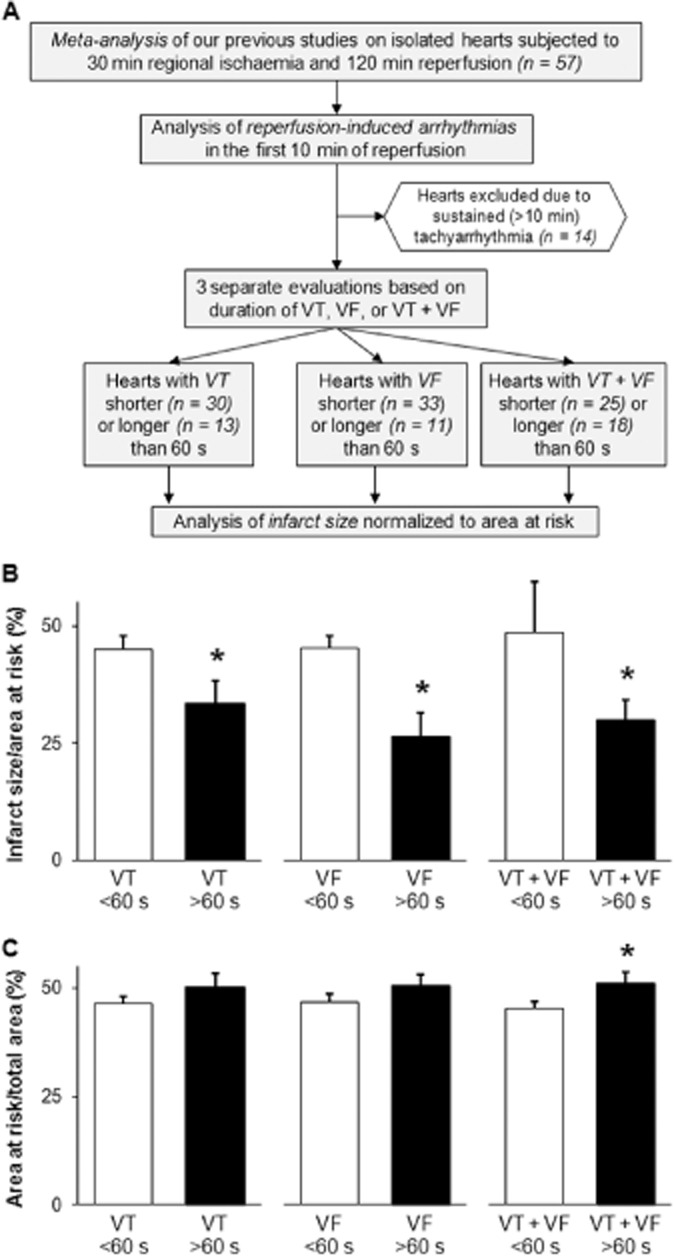
Duration of reperfusion-induced VT and/or VF is associated with decreased infarct size: a meta-analysis. Flow chart of the meta-analysis (A) indicates that reperfusion-induced tachyarrhythmias and infarct size data from our previous studies on isolated rat hearts subjected to 30 min regional ischaemia and 120 min reperfusion were analysed in three separate ways considering the duration of either VT, VF or both in the first 10 min of reperfusion. The results of the meta-analysis show infarct size normalized to area at risk (B) and area at risk (C) in the presence of shorter (<60 s) or longer (>60 s) total durations of VT, VF or VT + VF respectively. Values are expressed as mean ± SEM. *P < 0.05 versus corresponding <60 s groups, unpaired t-test.
Experimental design 1: testing the cardioprotective effect of RVP
To examine whether RVP applied at the onset of reperfusion induces cardioprotection, isolated hearts were perfused as shown in Figure 2A. Three experimental groups were designed: (i) I/R control; (ii) ischaemic postconditioning; and (iii) RVP groups (n = 12 in each group). The I/R control group was subjected to a 15 min equilibration period, followed by 30 min regional index ischaemia and 120 min reperfusion. IPost was induced by six consecutive cycles of 10 s reperfusion and 10 s no-flow global ischaemia at the onset of reperfusion. In the RVP group, the spontaneous rhythm of hearts was replaced by a 10 s pacing period (600 beats min−1; 10 Hz) in six alternating cycles during the first 2 min of reperfusion.
Figure 2.
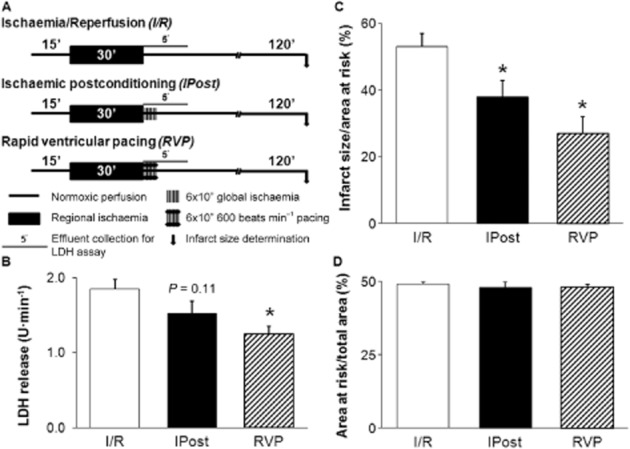
RVP reduces post-ischaemic LDH release and infarct size. Experimental protocol (A), post-ischaemic LDH release (B), infarct size normalized to area at risk (C) and area at risk (D). Hearts were subjected to a 15 min equilibration period, followed by 30 min regional ischaemia and 120 min reperfusion. Ischaemic postconditioning was induced by 6 × 10 s/10 s cycles of reperfusion/no-flow global ischaemia. In the RVP group, the autonomic rhythm of the hearts was replaced by 10 s pacing period (600 beats min-1; 10 Hz) in six alternating cycles at the onset of reperfusion. Coronary effluent was collected during the first 5 min of reperfusion for LDH activity determination (n = 5 in each group), the measured activities were multiplied by the corresponding coronary flow to give LDH release. Infarct size was measured at the end of reperfusion (n = 12 in each group). Values are expressed as mean ± SEM. *P < 0.05 versus I/R, one-way anova.
To assess the severity of cellular damage in the myocardium, the activity of LDH enzyme from coronary effluents (collected during the first 5 min of reperfusion) was measured using an LDH-P kit (Diagnosticum, Budapest, Hungary) (n = 5 in each group). The enzyme activity (U·mL−1) measured in an effluent was multiplied by the corresponding coronary flow (mL·min−1) to give LDH release expressed as U·min−1.
To determine infarct size, the LAD was re-occluded at the end of reperfusion and hearts were stained with 0.1% Evans blue to determine area at risk (Csonka et al., 2010). Hearts were then frozen at −20°C and cut into approximately 2 mm thick slices. Each slice was incubated at 37°C for 10 min in 1% 2,3,4-triphenyl-tetrazolium-chloride solution dissolved in phosphate buffer (pH 7.4). Slices were then fixed in 10% formaldehyde and scanned. Infarct size was evaluated by planimetry (InfarctSize™ 2.4.b; Pharmahungary Group, Szeged, Hungary) and normalized to area at risk.
To assess reperfusion-induced tachyarrhythmias (VT and VF), ECG was recorded (Isosys; Experimetria Inc.) during the entire perfusion protocol. Analysis of arrhythmias was carried out according to the original Lambeth conventions (Walker et al., 1988).
Experimental design 2: investigating the role of peroxynitrite and possible downstream targets in RVP-induced postconditioning
To assess the possible role of peroxynitrite in cardioprotection induced by ischaemic- or RVP-induced postconditioning, in separate experiments, cardiac 3-nitrotyrosine, a well-known peroxynitrite marker, was determined. To confirm increased peroxynitrite formation, cardiac superoxide anion was also measured. Furthermore, involvement of molecular mechanisms (i.e. RISK and SAFE pathways, HO1) that have been implicated in cardioprotection (Hausenloy and Yellon, 2004; Lecour, 2009; Bak et al., 2010) was also investigated as possible downstream targets of RVP-induced postconditioning.
Hearts were subjected to 15 min equilibration period, followed by 30 min regional ischaemia and 7 min reperfusion with or without IPost or RVP (Figure 4A). At the end of reperfusion, myocardial samples were taken from the ischaemic zone of the left ventricle for 3-nitrotyrosine measurement and Western blot analysis (n = 5 in each group). Sampling was carried out by an oblique cut from the origin of the LAD towards the right side of the apical area that involves the majority of the anterior wall of the left ventricle as well as the apex of the heart. Samples were rapidly freeze-clamped, powdered with a pestle and mortar in liquid nitrogen and stored in cryovials at −80°C until further analysis. Sampling for in situ detection of superoxide anion was carried out in separate experiments (n = 3 in each group) using the same perfusion protocol (Figure 4A). Approximately 3 mm thick transverse slices were cut from the middle of the ventricles, embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Zoeterwoude, The Netherlands), carefully frozen in isopentane pre-cooled in liquid nitrogen and stored at −80°C until sectioning with a microtome.
Figure 4.
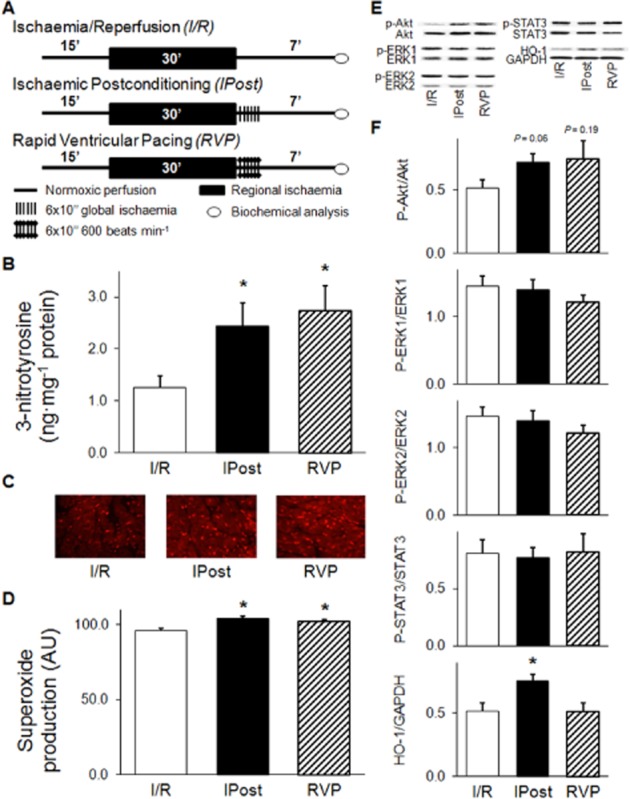
Postconditioning by RVP enhances formation of peroxynitrite and superoxide anion, effects on possible downstream targets. Experimental protocol (A), level of free cardiac 3-nitrotyrosine (B), representative images of in situ superoxide detection (C), quantification of in situ superoxide anion level (D), representative images (E) and quantification (F) of Western blots of possible downstream targets. Hearts were subjected to a 15 min equilibration period, followed by 30 min of regional ischaemia and 7 min reperfusion with or without ischaemic postconditioning or RVP. At the end of reperfusion, myocardial samples were taken from the ischaemic zone of the left ventricle for biochemical analysis. The peroxynitrite marker, 3-nitrotyrosine, was quantified by elisa (n = 5 in each group). Transverse cardiac sections from three hearts per group were used for in situ detection of superoxide anion (n = 60 random images in each group). Activation of RISK (Akt, ERK1/2) and SAFE (STAT3) pathways as well as protein level of HO1 was assessed by Western blot. Values are expressed as mean ± SEM. *P < 0.05 versus I/R, one-way anova. p-Akt, phospho(Ser473)-Akt; p-ERK1, phospho(Thr202)-ERK1; p-ERK2, phospho(Tyr204)-ERK2; p-STAT3, phospho(Tyr705)-STAT3.
Cardiac free 3-nitrotyrosine content, a marker of peroxynitrite, was measured by elisa (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions (Kupai et al., 2009; Kocsis et al., 2012). Briefly, homogenates were incubated overnight with nitrotyrosine acetylcholinesterase tracer and anti-nitrotyrosine rabbit IgG in microplates pre-coated with mouse anti-rabbit IgG. Ellman's reagent was used for development. Free nitrotyrosine content was normalized to protein content of cardiac homogenate and expressed as ng mg=1 protein.
Superoxide anion (O2−) is a reactive oxygen radical that reacts with NO to form peroxynitrite. The in situ fluorescent dihydroethidium staining was performed to evaluate intracellular production of superoxide anion (Varga et al., 2013). Unfixed frozen heart sections (30 μm) were placed on glass slides and incubated in 10−6 mol·L−1 dihydroethidium (Sigma, St. Louis, MO, USA) in PBS buffer (pH 7.4) at 37°C for 30 min in a dark humidified container. Fluorescence was then detected by a fluorescent microscope (Nikon, Tokyo, Japan) with a 590 nm long-pass filter. Images of the hearts were collected digitally (n = 20 in each heart); integrated density was evaluated by ImageJ 1.44p software and expressed in arbitrary unit.
The involvement of possible downstream targets in the mechanism of RVP-induced postconditioning was examined by standard Western blot techniques (Kocsis et al., 2008; Fekete et al., 2013). Tissue samples were homogenized with an ultrasonicator (UP100H Hielscher, Teltow, Germany) in RIPA buffer [50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 0.5% sodium deoxycholate, 5 mM EDTA, 0.1% SDS, 1% NP-40] supplemented with protease inhibitor cocktail (Sigma), PMSF, NaF and Na3VO4. The crude homogenates were centrifuged at 10 000× g for 10 min at 4°C. After quantification of protein concentrations of the supernatants using BCA Protein Assay Kit (Pierce, Rockford, IL, USA), 20 μg (50 μg for HO1) reduced and denaturated protein was loaded and SDS-PAGE (10% gel, 90 V, 1.5 h) was performed followed by transfer of proteins onto nitrocellulose membrane (20% methanol, 35 V, 2 h). Membranes were blocked for 1 h in 5% (w v−1) BSA at room temperature and then incubated with primary antibodies against phospho(Ser473)-Akt 1:500, Akt 1:2000, phospho(Thr202/Tyr204)-ERK1/ERK2 1:2000, ERK1/ERK2 1:1000, phospho(Tyr705)-STAT3 1:2000, STAT3 1:2000 (Cell Signaling, Beverly, MA, USA; overnight, 4°C, 5% BSA) or HO1 1:2000 (Enzo Life Sciences, Plymouth Meeting, PA, USA; 2 h, room temperature, 1% milk) or GAPDH 1:10 000 (Cell Signaling, Beverly, MA, USA; 1 h, room temperature, 1% milk). After incubation with HRP-conjugated secondary antibody 1:5000 (1:20 000 for GAPDH) (Dako Corporation, Santa Barbara, CA, USA; 1 h, room temperature, 1% milk), membranes were developed using an enhanced chemiluminescence kit (Pierce).
To further prove that both IPost and RVP protocols (i.e. application of brief I/R or RVP) facilitate peroxynitrite formation, 3-nitrotyrosine was measured in the absence of index ischaemia. The effect of the protocols on possible downstream targets of peroxynitrite (i.e. RISK and SAFE pathways) was also examined in the absence of preceding index ischaemia.
In this set of experiments, the time course of the perfusion protocol was adjusted to the previous set-up without index ischaemia (Figure 5A). In the normoxic perfusion group (n = 8), hearts were perfused for 52 min. In the repeated brief I/R group (n = 7), hearts were subjected to 45 min perfusion followed by 6 × 10/10 s cycles of no-flow global I/R and 5 min reperfusion. In the repeated brief RVP group (n = 8), the spontaneous rhythm of the hearts was replaced by 10 s pacing period (600 beats min−1; 10 Hz) in six alternating cycles after 45 min perfusion. At the end of perfusion, the cardiac free 3-nitrotyrosine level was determined and RISK as well as SAFE pathways were examined as described earlier.
Figure 5.
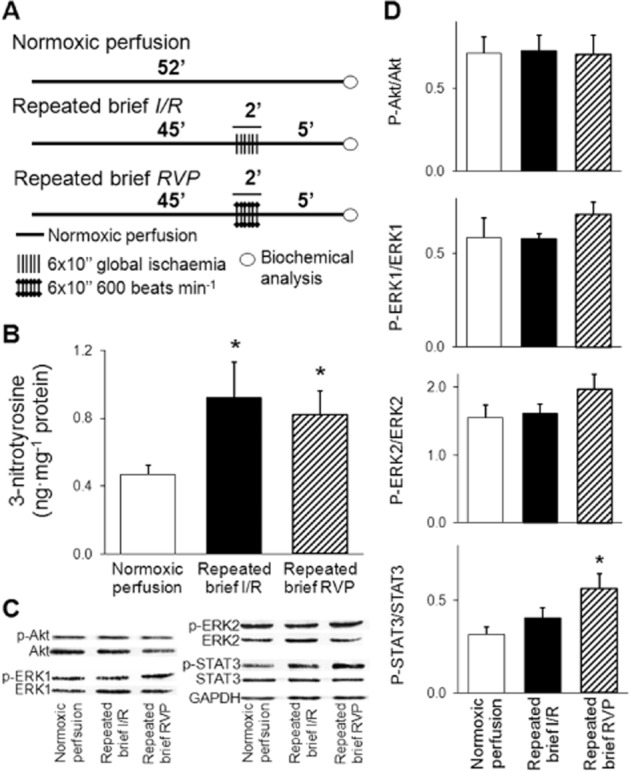
Postconditioning manoeuvres without a preceding index ischaemia enhance peroxynitrite formation, effects on possible downstream targets. Experimental protocol (A), level of free cardiac 3-nitrotyrosine (B), and representative images (C) and quantification (D) of Western blots of possible downstream targets. After 45 min normoxic perfusion, repeated (6 × 10/10 s) brief cycles of no-flow global I/R (n = 7) or RVP at 600 beats min-1/spontaneous rhythm of the hearts (n = 8) were applied followed by 5 min perfusion. In the normoxic perfusion control group (n = 8), hearts were perfused for 52 min. At the end of perfusion, cardiac free 3-nitrotyrosine level was determined by elisa and activation of RISK and SAFE pathways were examined by Western blots. Values are expressed as mean ± SEM. *P < 0.05 versus normoxic perfusion control, one-way anova. p-Akt, phospho(Ser473)-Akt; p-ERK1, phospho(Thr202)-ERK1; p-ERK2, phospho(Tyr204)-ERK2; p-STAT3, phospho(Tyr705)-STAT3.
Statistical analysis
Data are expressed as mean ± SEM and analysed by use of Student's unpaired t-test, one-way anova, or Fisher's exact test as appropriate. If a difference was established in anova, Fisher's least significant difference post hoc test was applied. Differences were considered significant at P < 0.05.
Results
Duration of reperfusion-induced VT and/or fibrillation is associated with decreased infarct size
Meta-analysis of six separate studies previously performed in our laboratory using the same experimental protocol (i.e. isolated rat hearts subjected to I/R) showed that the presence of VT, VF or VT + VF with a total duration of longer than 60 s in the first 10 min of reperfusion was associated with a markedly decreased infarct size (Figure 1B) respectively. In this analysis, a larger area at risk was associated with longer than 60 s total duration of VT + VF (Figure 1C).
RVP exerts cardioprotective effect: limits the infarction and reperfusion-induced arrhythmias
In order to assess the possible cardioprotective effect of RVP, the extent of myocardial infarction (LDH release and infarct size) was measured and reperfusion-induced arrhythmias were analysed.
The post-ischaemic LDH release was significantly reduced by RVP (Figure 2B). IPost also reduced LDH release; however, the difference did not reach the level of statistical significance (Figure 2B). Infarct size was significantly decreased by both IPost and RVP (Figure 2C). There was no difference in the area at risk of either experimental group (Figure 2D).
The incidence of VT and VF was not affected significantly by IPost in our present study (Figure 3). In contrast, short periods of RVP decreased the incidence of reperfusion-induced VT without having a significant effect on VF (Figure 3).
Figure 3.
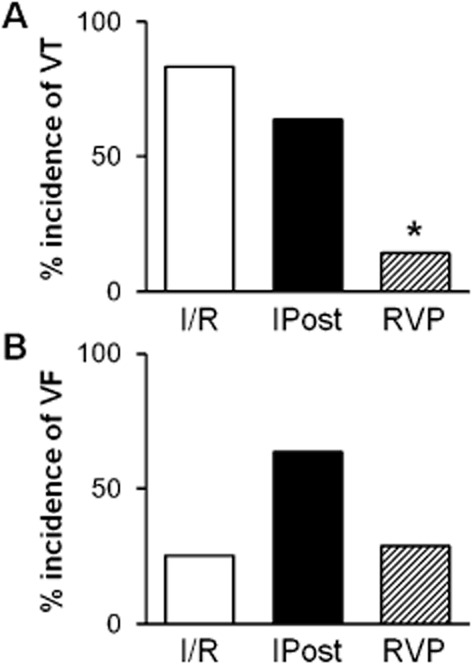
RVP attenuates reperfusion-induced arrhythmias. Incidence of reperfusion-induced VT (A) and VF (B) are shown. *P < 0.05 versus I/R, Fisher's exact test.
There was no difference in animal weight, heart wet weight, baseline heart rate and coronary flow (baseline, beginning of ischaemia, end of reperfusion) between the experimental groups (Table 2013). In contrast to IPost, coronary flow at the onset of reperfusion was not changed by short periods of RVP compared with I/R control (Table 2013).
Table 1.
Morphological and ex vivo haemodynamic parameters
| I/R | IPost | RVP | |
|---|---|---|---|
| Animal weight (g) | 367 ± 8 | 358 ± 10 | 345 ± 10 |
| Heart wet weight (g) | 1.28 ± 0.03 | 1.22 ± 0.04 | 1.30 ± 0.06 |
| Basal heart rate (beats min-1) | 301 ± 11 | 291 ± 12 | 304 ± 8 |
| Coronary flow (mL·min−1) | |||
| Before ischaemia | 18.8 ± 1.5 | 16.7 ± 1.2 | 18.7 ± 1.1 |
| Beginning of ischaemiaa | 10.7 ± 1.0 | 9.0 ± 0.8 | 11.5 ± 1.0 |
| Beginning of reperfusionb | 16.5 ± 1.0 | 8.7 ± 0.6* | 17.9 ± 0.7 |
| End of reperfusion | 11.5 ± 1.5 | 9.9 ± 0.9 | 11.8 ± 1.5 |
Results are expressed as mean ± SEM.
P < 0.05 versus I/R and RVP, one-way anova.
Regional ischaemia.
6 × 10 s global ischaemia was applied to induce IPost in the first 2 min of reperfusion. Coronary flow was measured by collecting coronary effluent for 2 min and then was expressed as mL·min−1.
Peroxynitrite is likely involved in RVP–induced postconditioning
To obtain some mechanistic insight into the beneficial effect of RVP, cardiac 3-nitrotyrosine and superoxide were measured at 7 min of reperfusion following the 30 min index ischaemia.
Postconditioning induced either by IPost or by RVP significantly increased free cardiac 3-nitrotyrosine level (a marker of peroxynitrite formation) (Figure 4B). Moreover, the peroxynitrite precursor superoxide anion was mildly, but significantly elevated in both postconditioning groups (Figure 4C).
To further prove that the postconditioning manoeuvres induce nitrative stress, cardiac 3-nitrotyrosine was measured after the postconditioning stimuli applied following normoxic perfusion without index ischaemia. The application of brief I/R cycles or periodic RVP increased the cardiac formation of 3-nitrotyrosine in the absence of index ischaemia (Figure 5B).
Downstream mechanisms of RVP-induced cardioprotection differs from that of ischaemic postconditioning
To elucidate the possible downstream targets of RVP, RISK and SAFE pathways as well as HO1 were investigated either in the presence or absence of index ischaemia.
Both postconditioning methods non-significantly enhanced Akt phosphorylation after index ischaemia at the beginning of reperfusion without affecting phosphorylation of ERK1/2 and STAT3 (Figure 4E, F). Protein level of HO1 was increased by IPost but not RVP (Figure 4E, F). In the absence of index ischaemia, applying short periods of RVP protocol increased STAT3 phosphorylation, in contrast to brief cycles of I/R (Figure 5C, D). Phosphorylation of Akt and ERK1/2 was not affected significantly by any of the interventions in the absence of index ischaemia (Figure 5C, D).
Discussion and conclusion
In our present study, using an isolated perfused rat heart model, we confirmed that IPost beneficially affects I/R injury. Moreover, we demonstrated for the first time in the literature that applying short periods of RVP at the onset of reperfusion also exerts a cardioprotective effect as it attenuates reperfusion injury by decreasing infarct size and reperfusion-induced arrhythmias. We showed that RVP increased peroxynitrite formation either in the presence or absence of index ischaemia in a way similar to IPost. These findings suggest that the formation of peroxynitrite in early reperfusion is a key event in the development of cardioprotection elicited by IPost or RVP. However, we also demonstrated that the downstream mechanisms of RVP-induced cardioprotection and IPost seem to be partially different.
In a meta-analysis of our previous studies on isolated hearts subjected to I/R, we analysed if there is an association between the duration of reperfusion-induced ventricular tachyarrhythmias (VT, VF or VT + VF) and infarct size. It is well accepted in the literature that I/R induces cellular damage that makes the myocardium more susceptible to arrhythmogenesis, and thus reperfusion-induced arrhythmias are considered as indicators of I/R injury (Engelen et al., 2003; Majidi et al., 2009). For instance, Majidi et al. reported that the presence of reperfusion arrhythmia bursts in STEMI patients is associated with a worse outcome (larger infarct size and decreased ejection fraction) (Majidi et al., 2009). However, here we found surprisingly that longer than 60 s reperfusion-induced VT/VF was associated with a decreased infarct size. In this analysis, a larger area at risk was associated with longer total duration of VT + VF in accordance with the literature data (Curtis and Hearse, 1989). The interpretation of these results is difficult since causality was not examined in these studies. A possible explanation for the results of our meta-analysis is that the size of infarction affects the occurrence of sustained VT and/or VF, while another possibility is that longer tachyarrhythmias at the beginning of reperfusion somehow attenuate infarct development. To the best of our knowledge, this latter approach has not been investigated in the literature and, therefore, these findings served as a basis for our current experimental study to investigate if exogenous application of controlled tachycardia induced by RVP at the onset of reperfusion is able to elicit cardioprotection.
Heart rate is known to play a role in the development of I/R injury (Bernier et al., 1989) and its controlled modification may elicit cardioprotection. For instance, pharmacologically-induced bradycardia (Tosaki et al., 1987), slow (Tosaki et al., 1988) or rapid (Ferdinandy et al., 1998; Hearse et al., 1999) pacing before ischaemia was reported to limit myocardial injury. Since the presence of longer reperfusion-induced tachyarrhythmias was associated with lower infarct size in our meta-analysis, we wanted to test whether exogenous rapid pacing exerts protection. To the best of our knowledge, we demonstrated for the first time in the literature that the application of short periods of rapid (600 beats min-1) ventricular pacing at the beginning of reperfusion reduces infarct size and reperfusion-induced arrhythmias.
In the present study, both RVP and classic IPost decreased infarct size. The beneficial effect of RVP on infarct size was further confirmed by a reduction in LDH release into the coronary effluent. Infarct size is a key determinant of major clinical outcomes (mortality and morbidity of consequent heart failure) (Gibbons et al., 2004); therefore, development of procedures that effectively decrease infarct size along with reperfusion therapy is in the focus of preclinical and clinical studies (Ovize et al., 2010). IPost is a widely studied approach, and the infarct size-reducing effect of this procedure was confirmed in various mice, rat, rabbit, dog and swine animal models (Skyschally et al., 2009b) as well as in clinical trials (Ovize et al., 2010). However, some studies reported the ineffectiveness of IPost in animal models (Dow and Kloner, 2007; Skyschally et al., 2009b) and in clinical trials (Hahn et al., 2013). A possible explanation for the controversial results could be that the cardioprotective effect of IPost depends upon several factors such as (i) species, strain, gender and age of research animal; (ii) experimental model and set-up; (iii) the duration of index ischaemia before reperfusion; (iv) number and duration of brief I/R cycles; (v) technical difficulty to achieve complete reperfusion; (vi) temperature; and (vii) presence of co-morbidities. These confounding factors indicate the necessity to develop alternative methods of IPost and we suggest that RVP-induced postconditioning is a simple method that eliminates technical problems associated with the induction of IPost.
Besides infarct size reduction, RVP-induced postconditioning decreased reperfusion-induced ventricular arrhythmias as well. Reperfusion therapy is accompanied by the occurrence of arrhythmias (Krumholz and Goldberger, 1991). Some of them are benign (e.g. accelerated idioventricular rhythm, the most common type) but others are potentially life-threatening malignant arrhythmias such as VT or VF that need to be managed in the clinical practice to avoid fatal consequences. Based on the literature data (Kloner et al., 2006), IPost effectively decreases ventricular arrhythmias. However, in our present study, solely RVP-induced postconditioning reduced the incidence of reperfusion-induced VT with no significant effect on VF. The reason for the inability of RVP to improve post-ischaemic VF is not clear. However, one may speculate that some interacting triggers of reperfusion-induced VF (e.g. reactive oxygen intermediates and calcium) may interfere with the possible anti-VF effect of RVP (Hearse and Tosaki, 1988).
Here, we demonstrated that IPost and RVP-induced postconditioning enhanced peroxynitrite formation at the onset of reperfusion after an index ischaemia. In addition, postconditioning manoeuvres themselves (i.e. brief I/R and RVP) increased peroxynitrite formation in the absence of the index ischaemia. Since peroxynitrite is reported as a possible trigger of IPost (Kupai et al., 2009), based upon our current results, we propose that the enhanced peroxynitrite formation also plays a role in triggering RVP-induced postconditioning. Back in 1997, Yasmin et al. reported that the level of peroxynitrite increases during reperfusion, which contributes to reperfusion injury in isolated rat hearts (Yasmin et al., 1997). Further studies also confirmed that enhanced peroxynitrite formation plays a central role in numerous cardiovascular diseases by inducing oxidative, nitrative and nitrosative stress (Pacher et al., 2007). However, peroxynitrite was demonstrated to have physiological functions (Lefer et al., 1997) and to play a role in triggering ischaemic preconditioning (Altug et al., 2000; Altup et al., 2001; Csonka et al., 2001). We have previously reported for the first time that peroxynitrite is a trigger of IPost since the peroxynitrite scavenger, FeTPPS, interfered with the cardioprotective effect of IPost (Kupai et al., 2009). Our results were confirmed by Li et al. showing that peroxynitrite is a key mediator of IPost in vivo (Li et al., 2013). Nevertheless, the possible mechanisms lying downstream of peroxynitrite formation in postconditioning have not been elucidated.
Here, we also looked at possible targets of endogenous peroxynitrite formation induced by IPost or by RVP. Several studies have reported that the activation of RISK (Akt, ERK1/ERK2) and SAFE (STAT3) pathways at the onset of reperfusion might play a role in the cardioprotective effect of IPost (Hausenloy, 2009; Lecour, 2009). In other studies, overexpression of HO1 was shown to reduce infarct size in the heart (Bak et al., 2010) and was implicated in pulmonary and hepatic IPost (Xia et al., 2009; Zeng et al., 2011). In our present study, both IPost and RVP-induced postconditioning non-significantly enhanced Akt phosphorylation without affecting ERK1/2 and STAT3 at the beginning of reperfusion. Although several studies showed increased phosphorylation of Akt and/or ERK due to IPost (Tsang et al., 2004; Yang et al., 2004), some recent papers suggested that postconditioning did not activate the RISK pathway in the early phase of reperfusion (Skyschally et al., 2009a; Fekete et al., 2013). We also found here that IPost, but not RVP, increased HO1 protein in the heart. This effect of IPost on HO1 is in agreement with the findings of others in the lung and liver (Xia et al., 2009; Zeng et al., 2011). We also examined the effect of postconditioning manoeuvres (i.e. repeated brief cycles of I/R or RVP) in the absence of a preceding index ischaemia and found no activation of the RISK pathway. In these experiments, STAT3 phosphorylation was increased only by short periods of RVP protocol. Taken together, our present results indicate that (i) the downstream mechanisms of RVP-induced cardioprotection and IPost are partially different; (ii) HO1 is probably not involved in the cardioprotective effect of RVP-induced postconditioning; and (iii) the precise role of the RISK and SAFE pathways remains to be elucidated in future studies. The involvement of alternative pathways in the protective effect of RVP-induced postconditioning is likely and may include, for instance, activation of NO-cGMP-PKG, sphingosine-, PKC- or CGRP-mediated pathways (Heusch et al., 2008; Bice and Baxter, 2014). Since endogenous NO-cGMP plays a role in protection against reperfusion injury by attenuating infarct size (Penna et al., 2006) and reperfusion-induced VF (Pabla et al., 1995; Pabla and Curtis, 1996), investigation of the exact role of NO in RVP would be interesting.
Although we clearly demonstrated that RVP induces cardioprotection when applied at the onset of reperfusion, some further limitations of our study may be considered. Firstly, ventricular pacing was reported to have direct pro-arrhythmic effects caused by the stimulus itself independently of the heart rate (Nakata et al., 1990). Although in our study ventricular pacing lasts only for short periods (6 × 10 s), and the incidence of reperfusion-induced VF was not increased in the RVP group when compared to I/R controls, consideration of pacing as an ectopic focus cannot be excluded. Secondly, in RVP-induced postconditioning, ventricles were activated in a non-physiological way in the present ex vivo study. Although the atrio-ventricular conduction system of rats was reported to be suitable for reaching 600 bpm heart rate by atrial pacing in an in vivo model (Gonzalez et al., 1998), further in vivo studies are needed to investigate the infarct size-limiting effect of postconditioning induced by rapid atrial or ventricular pacing at different rates. Thirdly, our study suggests that rapid heart rate at the early phase of reperfusion may contribute to initiation of adaptive molecular mechanisms to prevent I/R-induced cellular damage. However, further studies are needed to analyse (i) the precise molecular nature of these mechanisms and (ii) if reperfusion-induced spontaneous arrhythmias also trigger adaptive mechanisms in the myocardium. Our findings may also suggest that reperfusion-induced tachyarrhythmias require attention in future studies focusing on cardioprotection assessed by infarct size.
In conclusion, the application of short periods of RVP at the onset of reperfusion beneficially affects the essential components of reperfusion injury: the infarct size and reperfusion-induced ventricular arrhythmias. In addition, RVP increases peroxynitrite formation, which likely plays a role in triggering cardioprotection similarly to IPost. Nevertheless, downstream mechanisms in RVP-induced protection seem to be partially different from that of IPost, and further research is needed to elucidate them. Since RVP exerted a cardioprotective effect similar to IPost, we feel that RVP-induced postconditioning may serve as an alternative experimental model of IPost. Moreover, RVP could be performed in a more controlled manner than applying brief I/R cycles in IPost, which is an important technical advantage compared with IPost.
Acknowledgments
We are grateful to Nóra Bagi, Fatime Hawchar and Szilvia Török for their skilful technical assistance. We acknowledge the support of grants from the Hungarian Scientific Research Fund (OTKA K 79167), National Office for Research and Technology Grants (NKTH MED_FOOD, TÁMOP-4.2.1/B-09/1/KONV-2010-0005, TÁMOP-4.2.2.A-11/1/KONV-2012-0035). This work was also supported by János Bolyai Research Scholarship of the Hungarian Academy of Sciences (T. C. and C. C.).
Glossary
- HO1
haem oxygenase 1
- I/R
ischaemia/reperfusion
- IPost
ischaemic postconditioning
- LAD
left anterior descending coronary artery
- RISK
reperfusion injury salvage kinase
- RVP
rapid ventricular pacing
- SAFE
survival activating factor enhancement
- VF
ventricular fibrillation
- VT
ventricular tachycardia
Author contributions
Z. V. V. and T. C. designed the experiments. M. P., Z. V. V., K. K., G. F. K. and R. G. performed the research. M. P., Z. V. V. and C. C. analysed the data. C. C. and T. C. interpreted the data. M. P. drafted the manuscript. M. P., Z. V. V. and T. C. revised the manuscript. M. P., Z. V. V., K. K., R. G., G. F. K., C. C. and T. C. approved the final version of the manuscript.
Conflict of interest
Not declared.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altug S, Demiryurek AT, Kane KA, Kanzik I. Evidence for the involvement of peroxynitrite in ischaemic preconditioning in rat isolated hearts. Br J Pharmacol. 2000;130:125–131. doi: 10.1038/sj.bjp.0703280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altup S, Demiryurek AT, Ak D, Tungel M, Kanzik I. Contribution of peroxynitrite to the beneficial effects of preconditioning on ischaemia-induced arrhythmias in rat isolated hearts. Eur J Pharmacol. 2001;415:239–246. doi: 10.1016/s0014-2999(01)00843-3. [DOI] [PubMed] [Google Scholar]
- Bak I, Czompa A, Juhasz B, Lekli I, Tosaki A. Reduction of reperfusion-induced ventricular fibrillation and infarct size via heme oxygenase-1 overexpression in isolated mouse hearts*. J Cell Mol Med. 2010;14:2268–2272. doi: 10.1111/j.1582-4934.2010.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier M, Curtis MJ, Hearse DJ. Ischemia-induced and reperfusion-induced arrhythmias: importance of heart rate. Am J Physiol. 1989;256:H21–H31. doi: 10.1152/ajpheart.1989.256.1.H21. [DOI] [PubMed] [Google Scholar]
- Bice JS, Baxter GF. Postconditioning signalling in the heart: mechanisms and translatability. Br J Pharmacol. 2014;172:1933–1946. doi: 10.1111/bph.12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csonka C, Csont T, Onody A, Ferdinandy P. Preconditioning decreases ischemia/reperfusion-induced peroxynitrite formation. Biochem Biophys Res Commun. 2001;285:1217–1219. doi: 10.1006/bbrc.2001.5308. [DOI] [PubMed] [Google Scholar]
- Csonka C, Kupai K, Kocsis GF, Novak G, Fekete V, Bencsik P, et al. Measurement of myocardial infarct size in preclinical studies. J Pharmacol Toxicol Methods. 2010;61:163–170. doi: 10.1016/j.vascn.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Hearse DJ. Reperfusion-induced arrhythmias are critically dependent upon occluded zone size: relevance to the mechanism of arrhythmogenesis. J Mol Cell Cardiol. 1989;21:625–637. doi: 10.1016/0022-2828(89)90828-6. [DOI] [PubMed] [Google Scholar]
- Dow J, Kloner RA. Postconditioning does not reduce myocardial infarct size in an in vivo regional ischemia rodent model. J Cardiovasc Pharmacol Ther. 2007;12:153–163. doi: 10.1177/1074248407300897. [DOI] [PubMed] [Google Scholar]
- Engelen DJ, Gressin V, Krucoff MW, Theuns DA, Green C, Cheriex EC, et al. Usefulness of frequent arrhythmias after epicardial recanalization in anterior wall acute myocardial infarction as a marker of cellular injury leading to poor recovery of left ventricular function. Am J Cardiol. 2003;92:1143–1149. doi: 10.1016/j.amjcard.2003.07.020. [DOI] [PubMed] [Google Scholar]
- Fekete V, Murlasits Z, Aypar E, Bencsik P, Sarkozy M, Szenasi G, et al. Myocardial postconditioning is lost in vascular nitrate tolerance. J Cardiovasc Pharmacol. 2013;62:298–303. doi: 10.1097/FJC.0b013e3182993ae0. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Csont T, Csonka C, Torok M, Dux M, Nemeth J, et al. Capsaicin-sensitive local sensory innervation is involved in pacing-induced preconditioning in rat hearts: role of nitric oxide and CGRP? Naunyn Schmiedebergs Arch Pharmacol. 1997a;356:356–363. doi: 10.1007/pl00005062. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Szilvassy Z, Horvath LI, Csont T, Csonka C, Nagy E, et al. Loss of pacing-induced preconditioning in rat hearts: role of nitric oxide and cholesterol-enriched diet. J Mol Cell Cardiol. 1997b;29:3321–3333. doi: 10.1006/jmcc.1997.0557. [DOI] [PubMed] [Google Scholar]
- Ferdinandy P, Csonka C, Csont T, Szilvassy Z, Dux L. Rapid pacing-induced preconditioning is recaptured by farnesol treatment in hearts of cholesterol-fed rats: role of polyprenyl derivatives and nitric oxide. Mol Cell Biochem. 1998;186:27–34. [PubMed] [Google Scholar]
- Gibbons RJ, Valeti US, Araoz PA, Jaffe AS. The quantification of infarct size. J Am Coll Cardiol. 2004;44:1533–1542. doi: 10.1016/j.jacc.2004.06.071. [DOI] [PubMed] [Google Scholar]
- Gonzalez NC, Clancy RL, Moue Y, Richalet JP. Increasing maximal heart rate increases maximal O2 uptake in rats acclimatized to simulated altitude. J Appl Physiol (1985) 1998;84:164–168. doi: 10.1152/jappl.1998.84.1.164. [DOI] [PubMed] [Google Scholar]
- Hahn JY, Song YB, Kim EK, Yu CW, Bae JW, Chung WY, et al. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013;128:1889–1896. doi: 10.1161/CIRCULATIONAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ. Signalling pathways in ischaemic postconditioning. Thromb Haemost. 2009;101:626–634. [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Hearse DJ, Tosaki A. Free radicals and calcium: simultaneous interacting triggers as determinants of vulnerability to reperfusion-induced arrhythmias in the rat heart. J Mol Cell Cardiol. 1988;20:213–223. doi: 10.1016/s0022-2828(88)80054-3. [DOI] [PubMed] [Google Scholar]
- Hearse DJ, Ferrari R, Sutherland FJ. Cardioprotection: intermittent ventricular fibrillation and rapid pacing can induce preconditioning in the blood-perfused rat heart. J Mol Cell Cardiol. 1999;31:1961–1973. doi: 10.1006/jmcc.1999.1027. [DOI] [PubMed] [Google Scholar]
- Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner RA, Dow J, Bhandari A. Postconditioning markedly attenuates ventricular arrhythmias after ischemia-reperfusion. J Cardiovasc Pharmacol Ther. 2006;11:55–63. doi: 10.1177/107424840601100105. [DOI] [PubMed] [Google Scholar]
- Kocsis GF, Pipis J, Fekete V, Kovacs-Simon A, Odendaal L, Molnar E, et al. Lovastatin interferes with the infarct size-limiting effect of ischemic preconditioning and postconditioning in rat hearts. Am J Physiol Heart Circ Physiol. 2008;294:H2406–H2409. doi: 10.1152/ajpheart.00862.2007. [DOI] [PubMed] [Google Scholar]
- Kocsis GF, Sarkozy M, Bencsik P, Pipicz M, Varga ZV, Paloczi J, et al. Preconditioning protects the heart in a prolonged uremic condition. Am J Physiol Heart Circ Physiol. 2012;303:H1229–H1236. doi: 10.1152/ajpheart.00379.2012. [DOI] [PubMed] [Google Scholar]
- Krumholz HM, Goldberger AL. Reperfusion arrhythmias after thrombolysis. Electrophysiologic tempest, or much ado about nothing. Chest. 1991;99:135S–140S. doi: 10.1378/chest.99.4.135s. [DOI] [PubMed] [Google Scholar]
- Kupai K, Csonka C, Fekete V, Odendaal L, van Rooyen J, de Marais W, et al. Cholesterol diet-induced hyperlipidemia impairs the cardioprotective effect of postconditioning: role of peroxynitrite. Am J Physiol Heart Circ Physiol. 2009;297:H1729–H1735. doi: 10.1152/ajpheart.00484.2009. [DOI] [PubMed] [Google Scholar]
- Lecour S. Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: does it go beyond the RISK pathway? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Lefer DJ, Scalia R, Campbell B, Nossuli T, Hayward R, Salamon M, et al. Peroxynitrite inhibits leukocyte-endothelial cell interactions and protects against ischemia-reperfusion injury in rats. J Clin Invest. 1997;99:684–691. doi: 10.1172/JCI119212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Loukili N, Rosenblatt-Velin N, Pacher P, Feihl F, Waeber B, et al. Peroxynitrite is a key mediator of the cardioprotection afforded by ischemic postconditioning in vivo. PLoS ONE. 2013;8:e70331. doi: 10.1371/journal.pone.0070331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidi M, Kosinski AS, Al-Khatib SM, Lemmert ME, Smolders L, van Weert A, et al. Reperfusion ventricular arrhythmia ‘bursts’ predict larger infarct size despite TIMI 3 flow restoration with primary angioplasty for anterior ST-elevation myocardial infarction. Eur Heart J. 2009;30:757–764. doi: 10.1093/eurheartj/ehp005. [DOI] [PubMed] [Google Scholar]
- Miki T, Itoh T, Sunaga D, Miura T. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc Diabetol. 2012;11:67. doi: 10.1186/1475-2840-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Hearse DJ, Curtis MJ. Are reperfusion-induced arrhythmias caused by disinhibition of an arrhythmogenic component of ischemia? J Mol Cell Cardiol. 1990;22:843–858. doi: 10.1016/0022-2828(90)90116-j. [DOI] [PubMed] [Google Scholar]
- Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, et al. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- Pabla R, Curtis MJ. Endogenous protection against reperfusion-induced ventricular fibrillation: role of neuronal versus non-neuronal sources of nitric oxide and species dependence in the rat versus rabbit isolated heart. J Mol Cell Cardiol. 1996;28:2097–2110. doi: 10.1006/jmcc.1996.0202. [DOI] [PubMed] [Google Scholar]
- Pabla R, Bland-Ward P, Moore PK, Curtis MJ. An endogenous protectant effect of cardiac cyclic GMP against reperfusion-induced ventricular fibrillation in the rat heart. Br J Pharmacol. 1995;116:2923–2930. doi: 10.1111/j.1476-5381.1995.tb15946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl. Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna C, Cappello S, Mancardi D, Raimondo S, Rastaldo R, Gattullo D, et al. Post-conditioning reduces infarct size in the isolated rat heart: role of coronary flow and pressure and the nitric oxide/cGMP pathway. Basic Res Cardiol. 2006;101:168–179. doi: 10.1007/s00395-005-0543-6. [DOI] [PubMed] [Google Scholar]
- Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, et al. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009a;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- Skyschally A, van Caster P, Iliodromitis EK, Schulz R, Kremastinos DT, Heusch G. Ischemic postconditioning: experimental models and protocol algorithms. Basic Res Cardiol. 2009b;104:469–483. doi: 10.1007/s00395-009-0040-4. [DOI] [PubMed] [Google Scholar]
- Tosaki A, Szekeres L, Hearse DJ. Metoprolol reduces reperfusion-induced fibrillation in the isolated rat heart: protection is secondary to bradycardia. J Cardiovasc Pharmacol. 1987;10:489–497. doi: 10.1097/00005344-198711000-00001. [DOI] [PubMed] [Google Scholar]
- Tosaki A, Balint S, Szekeres L. Pacing and reperfusion induced arrhythmias: protection by slow heart rate in the rat heart. Cardiovasc Res. 1988;22:818–825. doi: 10.1093/cvr/22.11.818. [DOI] [PubMed] [Google Scholar]
- Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of ‘modified reperfusion’ protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- Varga ZV, Kupai K, Szucs G, Gaspar R, Paloczi J, Farago N, et al. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol. 2013;62:111–121. doi: 10.1016/j.yjmcc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Varga ZV, Zvara A, Farago N, Kocsis GF, Pipicz M, Gaspar R, et al. MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. Am J Physiol Heart Circ Physiol. 2014;307:H216–H227. doi: 10.1152/ajpheart.00812.2013. [DOI] [PubMed] [Google Scholar]
- Walker MJ, Curtis MJ, Hearse DJ, Campbell RW, Janse MJ, Yellon DM, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- Xia ZY, Gao J, Ancharaz AK. Protective effect of ischemic postconditioning on lung ischemia-reperfusion injury in rats and the role of heme oxygenase-1. Chin J Traumatol. 2009;12:162–166. [PubMed] [Google Scholar]
- Yamada M, Hearse DJ, Curtis MJ. Reperfusion and readmission of oxygen. Pathophysiological relevance of oxygen-derived free radicals to arrhythmogenesis. Circ Res. 1990;67:1211–1224. doi: 10.1161/01.res.67.5.1211. [DOI] [PubMed] [Google Scholar]
- Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44:1103–1110. doi: 10.1016/j.jacc.2004.05.060. [DOI] [PubMed] [Google Scholar]
- Yasmin W, Strynadka KD, Schulz R. Generation of peroxynitrite contributes to ischemia-reperfusion injury in isolated rat hearts. Cardiovasc Res. 1997;33:422–432. doi: 10.1016/s0008-6363(96)00254-4. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Zeng Z, Huang HF, Chen MQ, Song F, Zhang YJ. Contributions of heme oxygenase-1 in postconditioning-protected ischemia-reperfusion injury in rat liver transplantation. Transplant Proc. 2011;43:2517–2523. doi: 10.1016/j.transproceed.2011.04.021. [DOI] [PubMed] [Google Scholar]
- Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]


