Figure 1.
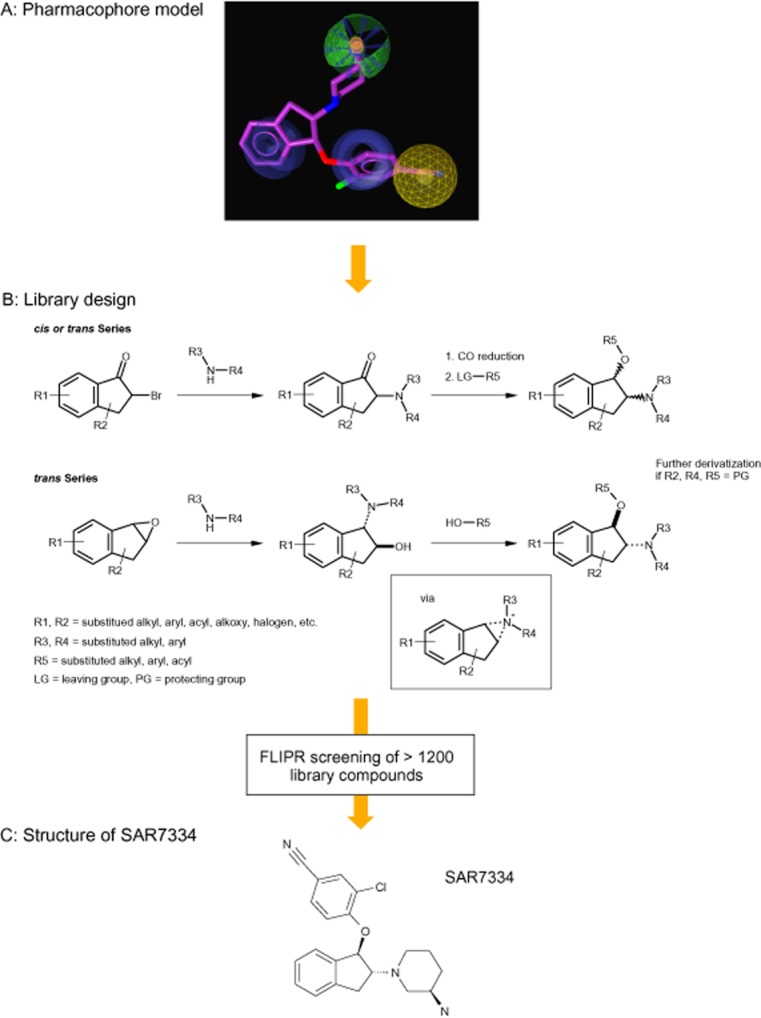
Discovery of SAR7334 using pharmacophore-guided design of focused aminoindanol libraries. Pharmacophoric information derived from a selection of known analogues of the cation channel inhibitor SKF96365 were translated into library designs and synthesized. Promising aminoindanol derivatives were further investigated with emphasis on stereochemical positioning of relevant substituents. (A) Mapping of SAR7334 to the TRPC6 pharmacophore model. The red sphere corresponds to a positive ionizable moiety, the light blue sphere indicates lipophilic features, while brown spheres mark ring aromatic features. The mapping of SAR7334 to the pharmacophore was generated with LigandScout (Inteligand Software-Entwicklungs und Consulting GmbH, Maria Enzersdorf, Austria). (B) Aminoindanol derivatives with cis or trans geometries were accessed from 2-bromo-1-indanones by nucleophilic substitution with amines, carbonyl reduction and subsequent O-alkylation/arylation. Trans geometries, in particular with aryloxy substituents (R5 = aryl) were realized by epoxide opening of indene oxide with amines and a Mitsunobu reaction with double inversion. (C) Structure of SAR7334.
