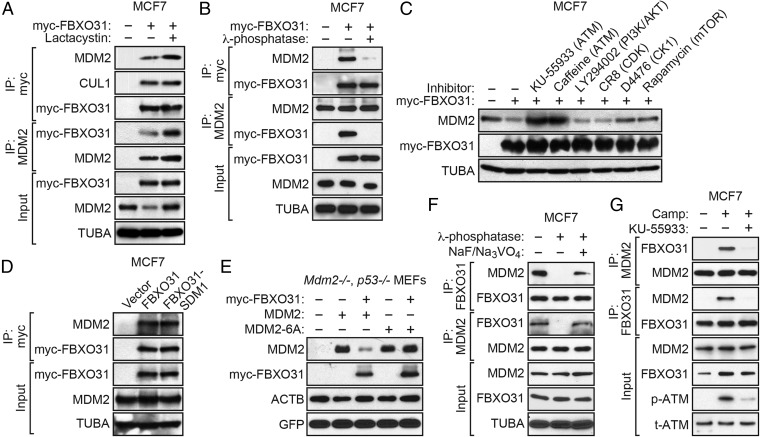
Fig. 3.
FBXO31 interacts with MDM2 directly in a manner that is dependent on phosphorylation of MDM2 by ATM. (A) Coimmunoprecipitation monitoring the interaction between ectopically expressed FBXO31 and endogenous MDM2 in MCF7 cells expressing vector or myc-FBXO31 and treated in the presence or absence of lactacystin. IP, immunoprecipitation. (B) Coimmunoprecipitation monitoring the FBXO31–MDM2 interaction in MCF7 cells treated in the presence or absence of λ-phosphatase. (C) Immunoblot monitoring MDM2 in MCF7 cells ectopically expressing FBXO31 and treated with kinase inhibitors. (D) Coimmunoprecipitation monitoring the FBXO31–MDM2 interaction in MCF7 cells expressing vector, myc-FBXO31, or myc-FBXO31-SDM1. (E) Immunoblot monitoring MDM2 in Mdm2−/−, p53−/− MEFs expressing vector or myc-FBXO31 and wild-type MDM2 or MDM2-6A. (F) Coimmunoprecipitation monitoring the endogenous FBXO31–MDM2 interaction in camptothecin-treated MCF7 cells in the presence or absence of λ-phosphatase or NaF/Na3VO4. (G) Coimmunoprecipitation monitoring the endogenous FBXO31–MDM2 interaction in MCF7 cells treated in the presence or absence of camptothecin or KU-55933.