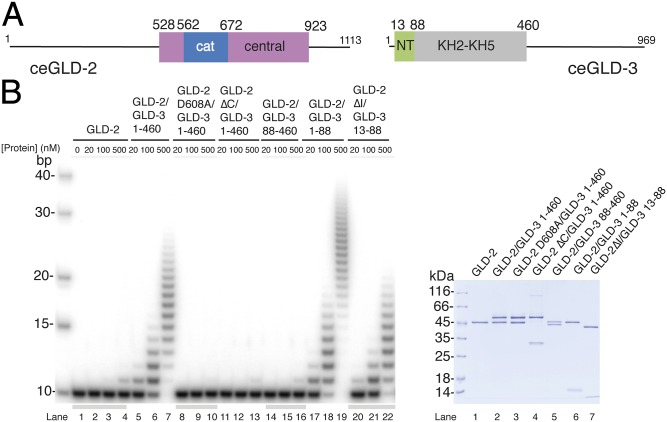
Fig. 1.
Poly(A)-polymerase core of the GLD-2–GLD-3 complex. (A) Schematic domain organization of C.e. GLD-2 and GLD-3. Folded domains are shown in rectangles and low-complexity sequences as lines. The portions of the molecule included in the structure reported in this work are in blue and pink [for the catalytic (cat) and central domains of GLD-2] and in green (the N-terminal NT domain of GLD-3). In gray is the folded region formed by the KH2-KH5 domains (34). (B) Poly(A)-polymerase assays with different C.e GLD-2 and GLD-3 protein fragments and mutants. In the assay, 20, 100, or 500 nM for proteins were incubated with 100 nM 5′-32P-labeled A10 RNA and 0.5 mM ATP. Reactions were run on a 10% polyacrylamide/7 M urea gel and visualized by phosphorimaging. The purified proteins used in the assay are shown at Right in a Coomassie-stained 12% SDS/PAGE gel.