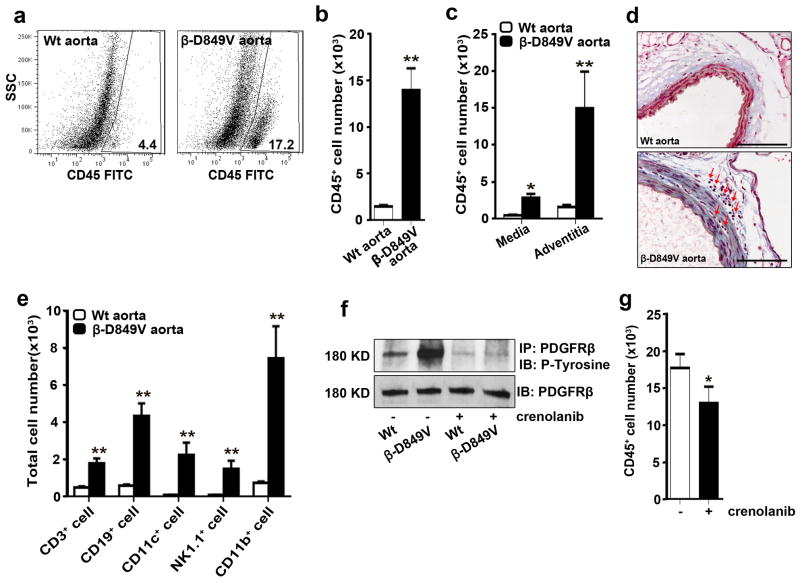
Figure 2. PDGF signaling in VSMCs causes inflammation of the aorta.
(a) Flow cytometric analysis of cells in the aorta of PDGFRβSm22D849V (β-D849V) mutant mice and Sm22α-Cre (Wt) control mice after enzymatic digestion. Numerical data indicate the percentage of CD45+ leukocytes among the total cells analyzed. Representative plots from three aortas of each genotype are shown. (b, c) Quantification of CD45+ leukocytes in the whole aorta or the separated media and adventitia as measured by flow cytometry, n=3 mice per genotype for each type of analysis. (d) Distribution of leukocytes (red arrows) in the media and adventitia was determined by staining cross sections of the thoracic aorta with Masson’s trichrome. Representative sections from 3 aortas of each genotype are shown. Scale bar, 100μm (e) Quantification of immune cell types in the aortic wall as measured by flow cytometry using fluorescent-conjugated antibodies: APC-CD3; APC.Cy7-CD19; PE-CD11c; PE.Cy7-NK1.1; PerCy5.5-Mac1, n=3 mice per genotype. (f) Phosphorylation of immunoprecipitated PDGFRβ from cultured VSMCs was determined by Western blotting with anti-phosphotyrosine antibody. PDGFRβ-D849V tyrosine kinase activity was inhibited by treatment with the PDGFR inhibitor Crenolanib (400ng ml−1) for 2 hours prior to cell lysis. Representative blot from 2 experiments is shown. (g) Quantification of CD45+ leukocytes in the whole aorta of PDGFRβSm22D849V mutant mice was measured by flow cytometry. Injecting mice with 15mg kg−1 Crenolanib for 5 consecutive days before sacrifice led to attenuation of CD45+ leukocytes in the aortic wall, n=3 mice per treatment. All data were assessed using Student’s t-test and are present as mean ± s.e.m. *P<0.05; **P<0.01.