FIG 5.
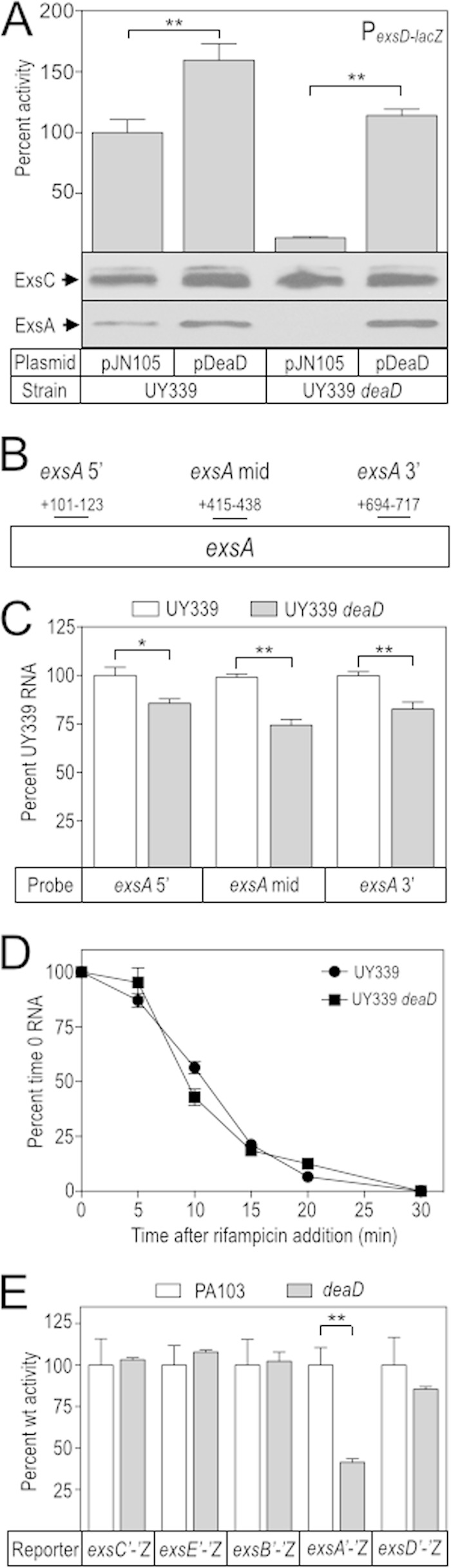
Dead controls ExsA synthesis at a posttranscriptional level. (A) The UY339 and UY339 deaD strains were cultured under inducing conditions for T3SS gene expression in the presence of 25 mM arabinose, and assayed for PexsD-lacZ reporter activity and ExsC and ExsA protein levels. The reported values were normalized to UY339 carrying pJN105 (389 Miller units). (B and C) Total RNA was harvested from the UY339 and UY339 deaD strains and exsA mRNA levels were measured at three independent locations within the ORF as indicated in panel B. We also measured rimM mRNA levels as an internal standard. The values reported in panel C were normalized to UY339 (100%) for each of the exsA probes. (D) The UY339 and UY339 deaD strains were cultured under inducing conditions for T3SS gene expression. When the culture A600 reached 1.0, the cells were treated with 200 μg of rifampin/ml, and RNA samples were then collected every 5 min over a 30-min period. The levels of exsA mRNA were measured using the exsA 5′ probe (B). The reported values were normalized to UY339 at time zero (100%). (E) exsC, exsE, exsB, exsA, and exsD. Expression of the reporters was controlled by a constitutive lacUV5 promoter, and the activity of each was determined in the wt PA103 and deaD backgrounds. The reported values were normalized to the activity of wt PA103 (100%) and reported as CPRG units: exsC′-′lacZ = 648, exsCE′-′lacZ = 100, exsCEB′-′lacZ = 384, exsCEBA′-′lacZ = 287, and exsD′-′lacZ = 306. *, P < 0.05; **, P < 0.005.
