Figure 3.
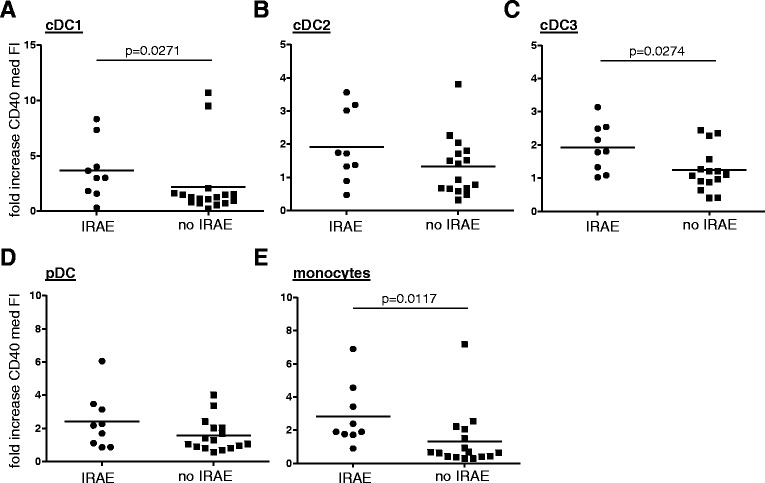
PBDC activation in relation to immune-related adverse events (IRAE). Fold increase in activation of A) cDC1, B) cDC2, C) cDC3, D) pDC and E) monocytes was determined at week 4 after start of prostate GVAX/ipilimumab treatment by dividing the median Fluorescence Index (med. FI) of CD40 at week four (i.e. 2 vaccinations and 1 ipilimumab infusion) by the med. FI of CD40 at start of treatment and displayed for patients that experienced IRAE or no IRAE during therapy. Differences in fold increase in activation between groups of patients were analyzed with the two-sample Mann-Whitney U test. Differences were considered significant when p < 0.05, as indicated with the given p-value.
