Figure 1.
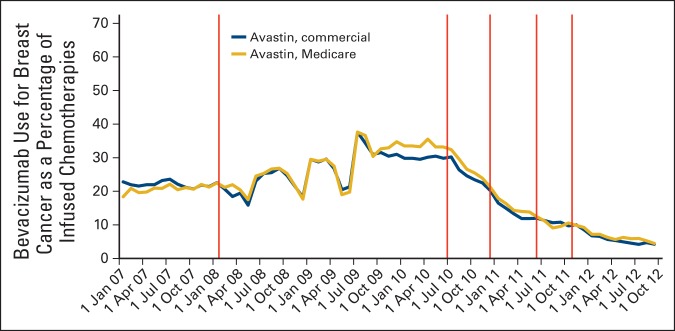
Trends in bevacizumab use for breast cancer as a proportion of infused chemotherapies by payer. Regulatory actions identified by vertical lines: February 2008, US Food and Drug Administration (FDA) approval for metastatic breast cancer. July 2010, FDA Oncologic Drugs Advisory Committee (ODAC) meeting on evidence supporting breast cancer indication withdrawal. December 2010, FDA announces intent to withdraw bevacizumab's breast cancer indication. June 2011, Second ODAC meeting. November 2011, FDA indicates withdrawal.
