Abstract
Liver transplantation was the product of 5 interlocking themes. These began in 1958-59 with canine studies of then theoretical hepatotrophic molecules in portal venous blood (Theme I) and with the contemporaneous parallel development of liver and multivisceral transplant models (Theme II). Further Theme I investigations showed that insulin was the principal, although not the only, portal hepatotrophic factor. In addition to resolving long-standing controversies about the pathophysiology of portacaval shunt, the hepatotrophic studies blazed new trails in the regulation of liver size, function, and regeneration. They also targeted inborn metabolic errors (e.g. familial hyperlipoproteinemia) whose palliation by portal diversion presaged definitive correction with liver replacement. Clinical use of the Theme II transplant models depended on multiple drug immunosuppression (Theme III, Immunology), guided by an empirical algorithm of pattern recognition and therapeutic response. Successful liver replacement was first accomplished in 1967 with azathioprine, prednisone, and ALG. With this regimen, the world’s longest surviving liver recipient is now 40 years postoperative. Incremental improvements in survival outcome occurred (Theme IV) when azathioprine was replaced by cyclosporine (1979) which was replaced in turn by tacrolimus (1989). However, the biologic meaning of alloengraftment remained enigmatic until multilineage donor leukocyte microchimerism was discovered in 1992 in long surviving organ recipients. Seminal mechanisms were then identified (clonal exhaustion-deletion and immune ignorance) that linked organ engraftment and the acquired tolerance of bone marrow transplantation and eventually clarified the relationship of transplantation immunology to the immunology of infections, neoplasms, and autoimmune disorders. With this insight, better strategies of immunosuppression have evolved. As liver and other kinds of organ transplantation became accepted as healthcare standards, the ethical, legal, equity, and the other humanism issues of Theme V have been resolved less conclusively than the medical-scientific problems of Themes I–IV.
The purpose of this contribution to the Master’s Perspective Series is to describe in detail the provenance of liver replacement. In the absence until now of such an account, liver transplantation often has been characterized as a natural extension of renal transplantation. In reality, liver and kidney transplantation were co-developed with the liver as the flagship organ, or alternatively the engine, for much of the time. In the process, the rising tide of organ transplantation altered the practice of hepatology, nephrology, and other organ-defined medical specialties, enriched multiple areas of basic and clinical science, and had pervasive ripple effects in law, public policy, ethics, and religion.
At first, liver transplantation was a fantasy. Transformation of the idea into a reality required the essentially de novo development between 1957 and 1962 of 5 separate but interconnected themes: (I) metabolic interactions between intra-abdominal organs (hepatotrophic physiology), (II) the liver and multivisceral transplant models including donor organ procurement and preservation, (III) the immune system and its control without or with therapeutic immunosuppression, (IV) transplantation outcomes, and (V) humanism-associated issues (social, ethical, legal, public policy).
The 5 themes can be used to categorize all of the liver transplant milestones of the last half century (1–71) as has been done by thematic color-coding and by numbers in Table 1. To help connect this history with the present and future, John Fung was recruited as a collaborating author, fresh from his 5-year tenure as Chief Editor of Hepatology’s sister journal, Liver Transplantation.
TABLE 1.
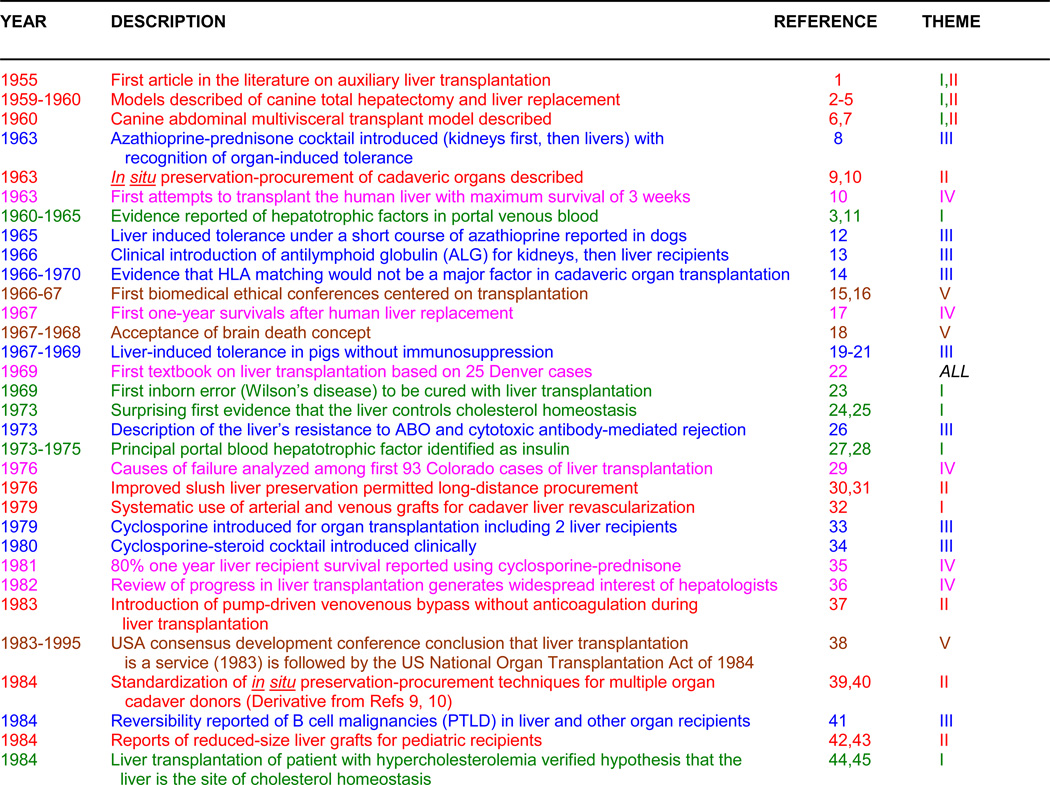 |
 |
1. Green: Hepatotrophic Physiology, 2. Red: Transplant Models, 3. Blue: Immunology, 4. Pink: Survival Results, 5. Brown: Humanism Issues
With major co-themes, the text color is of the dominant one
Abbreviations: UW = University of Wisconsin; PTLD = Post-transplant lymphoproliferative disorders
MY LIVERLESS EARLY LIFE
I was born in 1926 in the small town of LeMars, Iowa, and remained there uneventfully until joining the United States Navy directly from high school in 1944 (72). After the war’s end, I remained “in training” for 14 consecutive years, beginning at Westminster College (Fulton, Missouri), and continuing in chronologic order at the university medical centers of Northwestern, UCLA, Johns Hopkins, Miami, and again Northwestern. Tangible results from this period included PhD and MD diplomas (Northwestern, 1952), board certificates in general and thoracic surgery, and a dozen publications of which the first 5 were in neuroscience.
The Neuroscience Venture
My research on the brain stem circuitry of cats (and eventually monkeys) was started at Northwestern at the age of 23 years under the neurophysiology pioneer, Horace W. Magoun and finished at UCLA after Magoun’s recruitment there as one of the new school’s founding chairpersons. Each of the 5 resulting publications (73–77) generated 100 to 300 citations, and a figure from one (75) was immortalized as the logo of the UCLA Brain Institute. However, the Ph.D. thesis from this research and completion of the Northwestern M.D. requirements marked the end of my neurophysiology career at the age of 26 years.
The science environment that existed 60 years ago at both Northwestern and UCLA was described in my long letter of response in 1991 to a request by a UCLA Brain Institute archivist (see Supplementary Appendix #1). As described in that letter, Magoun’s influence cut deeply. He had no interest in, and very little tolerance for, research that did not have a clear mega-purpose. In our project, the global objective was to delineate with electrophysiologic technology the neural pathways serving the most fundamental elements of brain function: sleep versus wakefulness, cognition, and memory.
A Side Trip to Cardiac Physiology
The Supplementary Appendix #1 also contains a 1951 letter (discovered 4 decades later) from Magoun to Alfred Blalock, Chairman of Surgery at the Johns Hopkins Hospital, that undoubtedly contributed to my acceptance for surgical training at that great institution (1952–56). After completing the first year in Baltimore, I put aside all clinical work for 18 months to develop a model of complete heart block in dogs, a complication being caused in patients by efforts to close atrial or ventricular septal defects.
With the technology adapted from my neurophysiology experience, I showed that low voltage bipolar stimulation at any place on the ventricle was safe and efficient treatment for the bradycardia of heart block. The cardiac pacemaking was promptly instituted clinically at Hopkins and elsewhere. Although the articles describing the experimental work (78–80) also were frequently cited, my involvement in the subject of heart block now reached a dead end.
However, the youthful excursions were not wasted. What survived from my exposure to Magoun, and was evident in the heart block research, was the view that all biologic functions were products of a hierarchy of interacting systems and subsystems over which there were controls at multiple levels (i.e. regulatory brain equivalents). In this context, it was more important to learn how a given function was governed than to endlessly pursue details. The “big picture” approach (systems biology) would, in fact, be applied to liver transplantation, the third subject to which I directed concentrated attention.
THE SUCCESSION OF THEMES
Anatomically-influenced physiologic interactions between organs (Theme I)
While still at Johns Hopkins, I assisted Dr. Blalock perform a splenorenal shunt in a cirrhotic patient with insulin-dependent diabetes mellitus who then became insulin-free. The possibility that the portal diversion was responsible for the metabolic change seemed consistent with a then current hypothesis that excessive degradation of endogenous insulin during its primary passage to the liver via the portal vein was the cause of some forms of diabetes (81). Testing elements of this hypothesis was not possible until after I moved to the new medical school of the University of Miami to complete my general surgery residency (1956–58).
In Miami, I produced a colony of alloxan diabetic dogs, established the animals’ steady state insulin needs, and modified the liver’s blood supply with portacaval shunt (Eck’s fistula) or other alterations of the portal venous system (82,83). The objective of surgically ameliorating diabetes evaporated when the portal diversion procedures increased instead of decreasing the insulin requirements (83). In addition, the hepatic atrophy and systemic morbidity caused by portacaval shunt in normal dogs (84,85) appeared to be exaggerated in our diabetic animals.
Development of liver transplant models (Theme II)
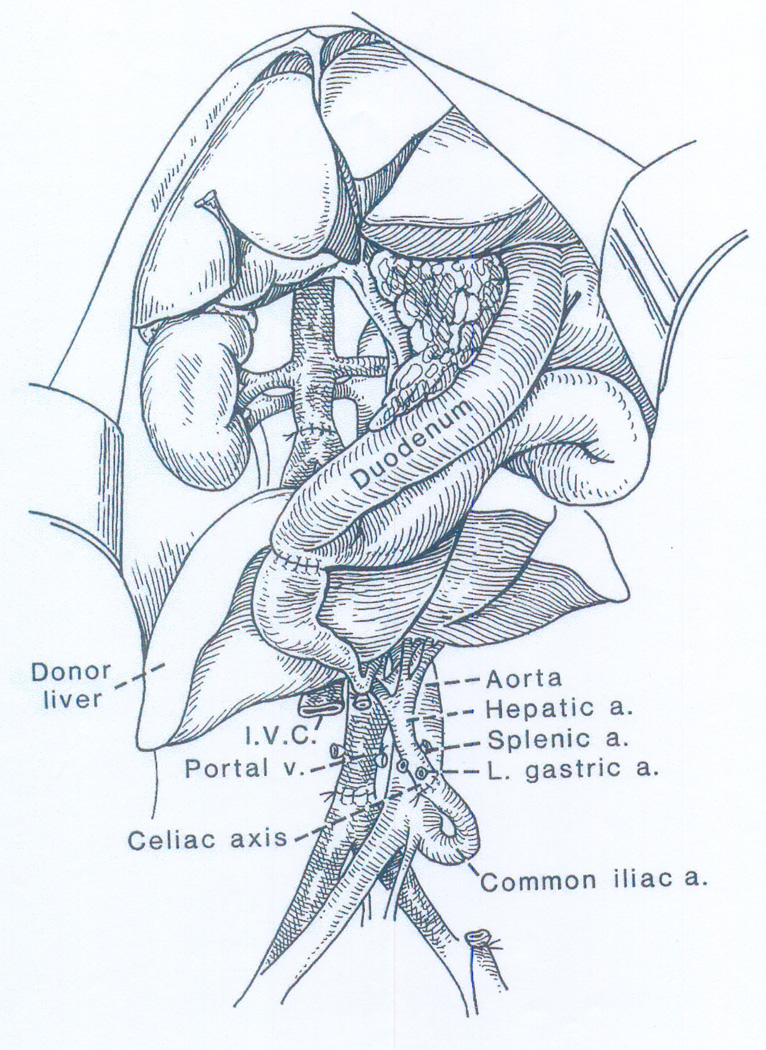
A connection of these studies to liver transplantation was made when C. Stuart Welch of Albany, New York, visited Miami in 1957 to give a lecture on the treatment of portal hypertension. During his talk, Welch made casual reference to a canine operation that he had reported in 1955 (1) and more extensively a year later (86). In these articles, the term “liver transplantation” was used for the first time in the scientific literature. The Welch operation consisted of revascularization of an auxiliary liver allograft in the recipient’s right paravertebral gutter with provision of portal venous inflow from the inferior vena cava (Figure 1).
Figure 1.
Auxiliary liver homotransplantation in dogs (the Welch procedure). Note that the portal venous inflow of the extra liver is from the inferior vena caval bed while the native liver retains a normal blood supply. It was suspected from the beginning that this was a major flaw in the design of the procedure. From: Ann Surg 160:411-439, 1964.
Recognizing that failure to provide the extra liver with a normal portal venous supply could handicap the allograft in the same way as the native livers were damaged in my non-transplant portal diversion models, I began the development of versatile transplant procedures to study the special qualities of splanchnic venous blood in dogs. One of the models was a method of total recipient hepatectomy, the unique feature of which was preservation of the retrohepatic inferior vena cava (2) as in the first stage of today’s piggy-back human liver transplantation. For liver allograft implantation, it was technically easier to simply remove this portion of the recipient vena cava and replace it with the comparable segment of the donor liver’s vena cava into which all of the hepatic veins empty (3).
Operative survival with the complete canine replacement operation (Figure 2) was not accomplished until a few days after I moved to Northwestern in June, 1958, for a final 12 months of cardiovascular surgical training that was expected to culminate in an academic practice in thoracic surgery. Instead, 2 steps were taken during the summer of 1958 that ensured pursuit of the liver research for at least 5 years beyond completion of the thoracic residency. The first step was the submission of a 4 page NIH grant focused on metabolic studies in which liver replacement was one of the experimental models. The second step was my nomination by Northwestern for a John and Mary Markle Scholarship. Here, the emphasis was radically different.
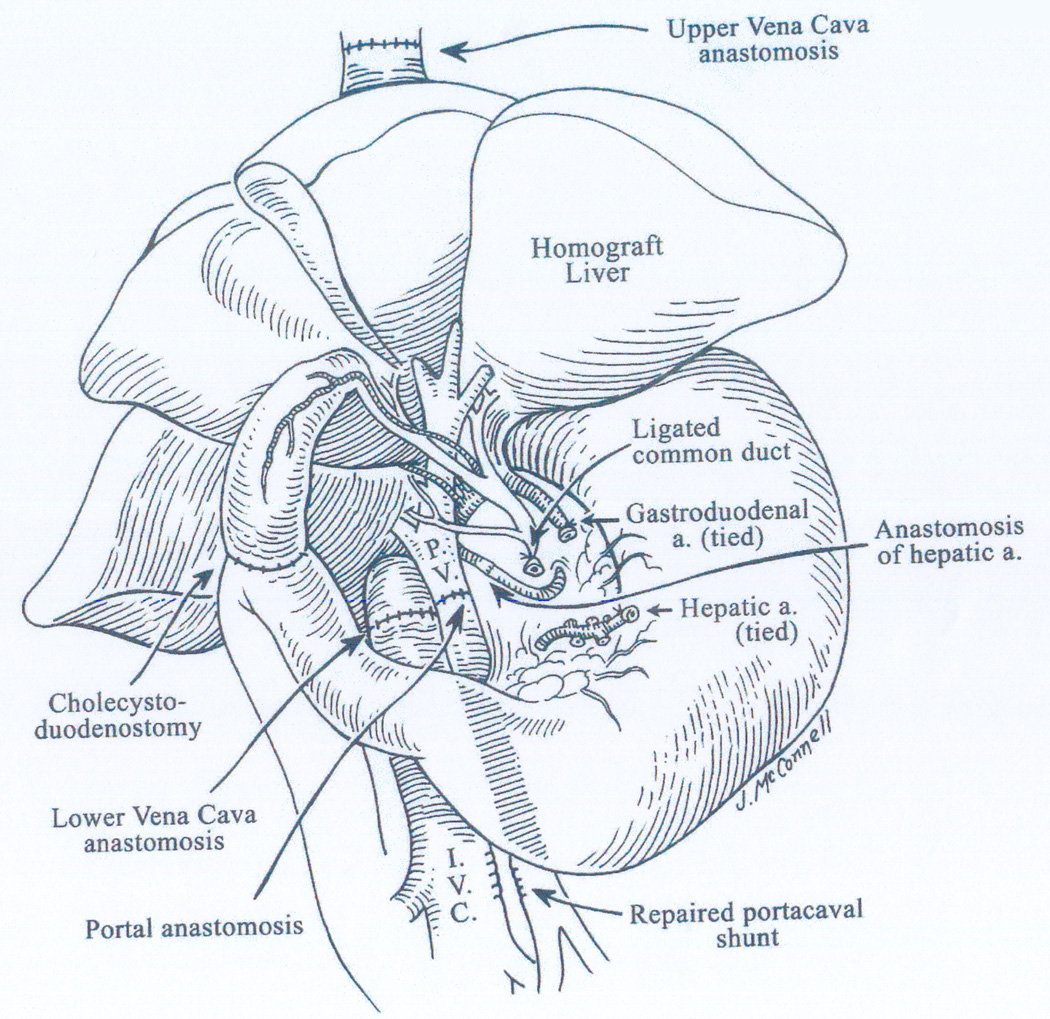
Figure 2.
Complete liver replacement in the dog circa 1958-9. The fact that this was a canine rather than a human operation is evident only from the small multiple lobes of the allograft and the biliary drainage with cholecystoduodenostomy. In my first report (3), an “outflow block” syndrome resembling endotoxin shock was described if donor body weight was less than half that of the recipient (one cause of today’s “small for size” syndrome).
Markle Scholar candidates were expected to identify an open-ended career objective. Ignoring advice to develop a “more realistic” project in the emerging field of open heart surgery, I proposed the life goal of clinical liver transplantation. In the autumn of 1958, I learned that the NIH grant would be fully funded for 5 years, and shortly thereafter that I had been selected as a Markle Scholar. The first phase of the canine liver project was nearly completed by the time I finished the thoracic residency and the dual revenue streams began on 1 July 1959. In addition, a second operation had been perfected in which the liver was transplanted as part of an allograft that contained all of the other intra-abdominal viscera (Figure 3) (6,7).
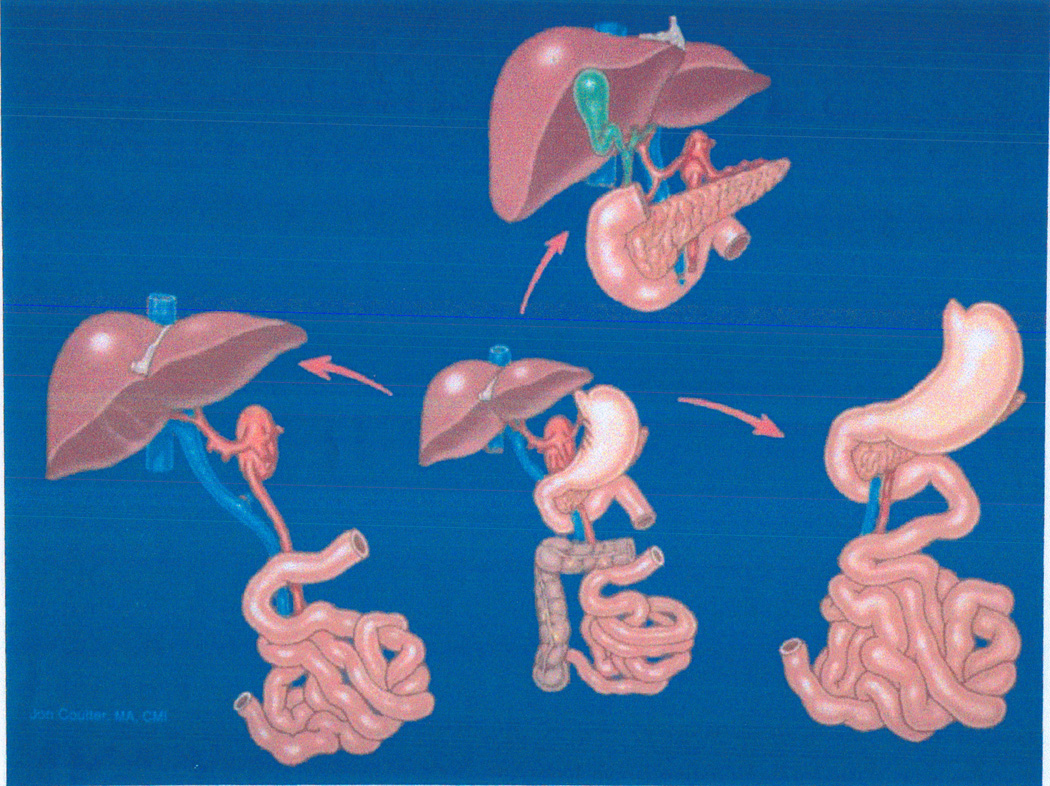
Figure 3.
Bottom Center: Multivisceral allograft transplanted in dogs in 1959 (6,7) and in humans for the first time 3 decades later (46). With removal of different organs from the common vascular stem, this original procedure has had many subsequent variations. Lower Left: Liver-intestinal transplantation (47,62). Top Middle: Cluster of upper abdominal organs (55). Right: Mid gut organs except the liver. From: Liver Transplant & Surg 4:1-14, 1998.
The magnitude of the Markle proposal should have been intimidating, but it did not seem so at the time. The slate of liver transplantation was nearly blank in 1958, but what had to be done was transparent: make the operation biologically sound, make it practical, and find a way to prevent allograft rejection. I was not the only person to think that way. Although I did not learn of it until a year later, Francis D. (Franny) Moore had begun independent efforts to replace the dog liver during the summer of 1958 at the Peter Bent Brigham Hospital in Boston (4,5) that continued until the mid-1960s (87,88).
Moore’s transplant interests were not confined to the liver. This can be perceived most clearly by reading his book, Give and Take (89) and his autobiography A Miracle and a Privilege (90) written 4 decades later. Epitomizing his ubiquitous presence, Moore presided as chief of surgery at the Brigham over the clinical renal transplant trials of Murray and Merrill that yielded the world’s first example in any species of > one year survival of an organ allograft (91). In this case, the kidney from a fraternal twin was transplanted to his irradiated brother on January 24, 1959, and functioned for the next 20 years without maintenance immunosuppression (Table 2).
TABLE 2.
CHARACTERISTICS OF THE FIRST SUCCESSFUL TRANSPLANTATION OF KIDNEY ALLOGRAFTS WITH >6 MONTHS SURVIVAL AS OF MARCH 1963
| PHYSICIAN | REF | SITE | DATE | DONOR RELATIONSHIP |
GRAFT SURVIVAL |
|---|---|---|---|---|---|
| Joseph E. Murray | 91 | Boston, Massachusetts |
January 24, 1959 | Fraternal twin | 20 yearsb |
| Jean Hamburger | 103 | Paris, France | June 29, 1959 | Fraternal twin | 26 yearsb |
| Rene Kuss | 105 | Paris, France | June 22, 1960 | Unrelated | 18 monthsb |
| Jean Hamburgera | 104 | Paris, France | December 19, 1960 | Mother | 22 monthsb |
| Rene Kussa | 105 | Paris, France | March 12, 1961 | Unrelated | 18 monthsb |
| Jean Hamburgera | 104 | Paris, France | February 12. 1962 | Cousin | 15 yearsc |
| Joseph E. Murray | 106,107 | Boston, Massachusetts |
April 5, 1962 | Unrelated | 17 monthsb,d |
| Thomas E. Starzl | 8,108–9 | Denver, Colorado |
1962–1963 | First Series: A mixture |
≥ 45 years |
Kuss and Hamburger described periodic administration of adrenal cortical steroids with these patients.
Patient death occurred at or shortly after listed time.
Patient underwent successful retransplantation in the 1970s; elected to French Parliament.
First successful with drugs-only immunosuppression (no radiation).
From my point of view, this faint signal that the genetic/immunologic barrier to organ alloengraftment might be surmountable made the liver transplant objective less distant. It seemed almost providential that the 5-year Markle Scholarship and NIH funding (1959–64) for my liver project began a few months after the fraternal twin transplantation. The 5 years was equally split between Northwestern where I was elevated to a junior faculty position on 1 July 1959, and the University of Colorado where I was appointed Associate Professor of Surgery and Chief Surgeon at the Denver VA Hospital from November 1961.
The Immune System and Its Control (Theme III)
Until 1958-60 the only organ allograft whose unmodified rejection had been thoroughly studied was the kidney. Rejection to death of our canine liver recipients usually occurred in 5 to 10 days (3). However, in rare outlyers in which the biochemical indices of rejection improved spontaneously, the liver allograft’s dominant histopathologic findings by 3 weeks were those of repair and regeneration (92). These were the first recorded exceptions to the existing dogma (based on skin graft research) that rejection, once started, was inexorable.
In the multivisceral grafts (Figure 3), the pathology was subtly different. Rejection of the various organs if they were part of the multivisceral graft was less severe than when the organs were transplanted alone. Moreover, there was overt evidence in recipient tissues of a graft versus host (GVH) reaction, but without a skin rash or other manifestations of graft versus host disease (GVHD) (7). The double immune reaction (host versus graft [HVG] and graft versus host) exposed by those experiments was shown a third of a century later to be a feature of alloengraftment and acquired tolerance no matter what the transplanted organ (see later).
Both my liver-alone and multivisceral transplant models were generally viewed as technical exercises of little if any scientific interest. One reason was the prevailing view that was concisely expressed in 1961 by the 1960 Nobel Laureate, F.M. Burnet in a New England Journal of Medicine review entitled, The New Approach to Immunology. The discouraging passage read: “… Much thought has been given to ways by which tissues or organs not genetically and antigenically identical with the recipient might be made to survive and function in the alien environment. On the whole, the present outlook is highly unfavorable to success” (93).
I was poorly equipped to rebut this kind of opinion. My attempts in Chicago to use radiation therapy for canine liver transplantation in 1959-60 failed miserably (94). During this bleak time, however, it was reported in a closely-spaced succession of articles that 6 mercaptopurine and/or its analogue, azathioprine, were immunosuppressive in non-transplant (95,96), rabbit skin graft (97,98), and canine kidney transplant models (99,100). The most extensive kidney transplant experiments were done by the 30 year old English surgeon, Roy Calne (101) who began his studies at the Royal Free Hospital in London in 1959 while still a registrar (resident). The work was continued in Boston with Joseph Murray after July 1960 (102).
In 1961, Calne visited our laboratory in Chicago and described his results. Shortly thereafter, I moved to Colorado, after making the decision to develop a human kidney transplant program there with drug immunosuppression as a forerunner for the liver objective. This would be a bold step since the renal center at the Brigham was the only one in America at the time with an active clinical transplant arm. After demonstrating in parallel canine kidney and liver transplant studies of azathioprine that advances with either organ would be applicable to the other, we concentrated our immunosuppression research on the simpler kidney model. Our most promising results were obtained by giving daily doses of azathioprine monotherapy before as well as after kidney transplantation, adding postoperative prednisone only when overt rejection developed.
By the time the incremental drug protocol was taken to the clinic in the autumn of 1962, 6 renal allograft recipients treated primarily or exclusively with the total body irradiation protocol of Murray’s fraternal twin case (see earlier) had either passed or would soon reach the one year survival milestone, including 2 French patients to whom the donors were not genetically related (Table 2) (91,103–105). In addition, Murray had transplanted a deceased donor allograft in Boston on April 5, 1962, under azathioprine-based immunosuppression (106,107). The kidney was destined to function for 17 months and become the world’s first to survive > 1 year with a radiation-free (drugs-only) protocol. Enthusiasm generated by this last case was tempered, however, by the fact that the recipient was the only one of the first 10 in the Boston azathioprine series to survive longer than 6 months (details annotated in Ref 108).
Some members of our Denver team concluded from this sobering news that our accrual of more renal transplant cases would be a futile and embarrassing undertaking. My counter argument was that our laboratory-based treatment strategy differed in many ways from the one used in the Boston protocol, including a role of prednisone equal in importance to that of azathioprine. The differences proved to be crucial. First in dogs, and then in human kidney recipients, the graded use of azathioprine and prednisone exposed the 2 features of the alloimmune response that provided the basis for the transplantation of all kinds of organs.
The 2 phenomena were capsulized in the title of a 1963 report of the first-ever series of successful kidney allotransplantations: “The reversal of rejection in human renal homografts with subsequent development of homograft tolerance” (8). The principal evidence that the allografts (then called homografts) had somehow induced variable donor specific tolerance was that the reversal of rejection frequently was succeeded by a time-related reduction, or in some cases elimination, of the need for maintenance immunosuppression. In fact, 8 recipients in the 1962-64 Colorado series of 64 still bear the world’s longest functioning renal allografts, 45 or more years later (109). Six of the 8 have been off all immunosuppression medications for 12 to 46 years.
Transplantation Outcomes With the Forerunner Kidney (Theme IV)
The > 70% one year patient and renal graft survival in our seminal Colorado series (110,111) exceeded my own expectations, and was not considered to be credible until David Hume in Richmond and others added their confirmatory experience. The world-wide reaction was remarkable. In the spring of 1963, there had been only 3 clinically active renal transplant centers in North America (Boston, Denver, and by now Richmond) and scarcely more in Europe. One year later, 50 new renal programs in the United States alone were either fully functional or were gearing up.
In reflecting back a dozen years later on the kidney transplant revolution of 1962-64, I began my founding lecture for the American Society of Transplant Surgeons (ASTS) with the comments that: “From time to time, a news story appears about the birth of a husky, full-term baby, much to the amazement of the chagrined mother who had not realized that she was pregnant. Mother surgery seemed to have been thus caught by surprise when clinical transplantation burst upon the scene in the early 1960s” (112).
Issues of humanism (Theme V)
Liver transplantation was swept up in the 1962-64 kidney momentum. However, there were many reasons to be cautious, not the least of which were social, ethical, and legal concerns. Throughout 1962, I discussed these issues personally with key non-university persons: the Colorado Governor (John Love), our United State Senator (Gordon Allot), the Denver Coroner, the Chief Justice of the Colorado Supreme Court, and clerical leaders. All ultimately expressed support. Resistance within the University was dealt with by the legendary medical school dean, Robert J. Glaser, and the University Chairman of Surgery, William R. Waddell.
Unprecedented technical challenges were expected. The liver replacement operation, which was difficult even under the optimal circumstances of the animal laboratory, predictably would be harder in recipients with portal hypertension and other pathophysiologic and anatomic changes of chronic liver disease. In the absence of artificial organ support, failure of the hepatic graft to promptly function would be tantamount to death. Finally, how could immediately life-supporting deceased donor livers be obtained in an era in which death was defined as the cessation of heart beat and respiration?
These questions and issues mandated consideration of the less draconian auxiliary hepatic transplant operation of Welch that might allow recipient survival, even if the graft failed. This option was undermined when the rapid atrophy of auxiliary livers that previously had been ascribed to rejection in unmodified dogs (86,113), was shown to be equally severe in animals in which rejection was prevented with azathioprine (11). The die was cast for the liver replacement (orthotopic) option.
THE FIRST HUMAN LIVER TRANSPLANTATIONS
Liver replacement was carried out in 7 deceased donor liver recipients between March 1963 and January 1964: 5 in Denver (cases 1–4 and 6), Boston (case 5 by Moore’s team) and Paris (case 7) (Table 3) (10,11,88,114). All 7 patients died, 2 during the operation and the other 5 after 6.5 to 23 days. Neither primary non-function nor uncontrolled rejection of the grafts were lethal factors in any of the failures.
TABLE 3.
FIRST RECIPIENTS OF REPLACEMENT LIVERS
| AGE | DATE | CITY (REF) | LIVER DISEASE |
SURVIVAL (DAYS) |
MAIN CAUSE OF DEATH |
|---|---|---|---|---|---|
| 3 | 3-1-63 | Denver (10) | Biliary Atresia | 0 | Intra-op Bleeding |
| 48 | 5-5-63 | Denver (10) | Hepatoma, Cirrhosis | 22 | Pulmonary Emboli, Sepsis |
| 68 | 6-3-63 | Denver (10) | Duct Cell Carcinoma | 7.5 | Pulmonary Emboli |
| 52 | 7-10-63 | Denver (11) | Hepatoma, cirrhosis | 6.5 | GI bleeding, pulmonary emboli/edema, liver failure |
| 58 | 9-16-63 | Boston (88) | Colon metastases | 11 | Pneumonitis, hepatic abscesses, failure |
| 29 | 10-4-63 | Denver (11) | Hepatoma | 23 | Sepsis, bile peritonitis, pulmonary emobli |
| 75 | 1-?-64 | Paris (114) | Colon metastases | 0 | Intraoperative hemorrhage |
At autopsy of the 4 Denver patients who survived the operation, pulmonary emboli were found that apparently had originated in the bypass tubing used to decompress the blocked systemic and splanchnic venous beds during the removal and replacement of the native liver. Ironically, the bypass which had been an essential component of the canine operation, is not mandatory in most human recipients, or even in dogs if venous collateralization is encouraged by bile duct ligation a month in advance (115).
By the time our fourth and fifth liver recipients were reported to the American Surgical Association in April 1964 (11), all clinical liver transplant activity had ceased in what would be a voluntary 3-1/2 year worldwide moratorium. The self-imposed decision to stop did little to quiet polite but unmistakably disapproving discussions of an operation that had come to be perceived as too difficult to ever be tried again.
THE MORATORIUM
In effect, it now would be necessary to return to ground zero and reexamine all 5 of the themes of Table 1. The central assumption of Theme I had been that portal venous blood contained hepatotrophic molecules. The hypothesis was consistent with our results in 1958-60 in non-immunosuppressed canine recipients of replacement livers (3), and especially with the acute atrophy of Welch’s auxiliary grafts in azathioprine-treated dogs (see earlier, and Ref 11). The possibility was now explored of providing the auxiliary allografts with direct access to the portal molecules (116).
But what were the hepatotrophic factors? Using double liver fragment non-transplant models derived from Welch’s auxiliary liver operation (Figure 4), it was proved during and after the moratorium that insulin is the principal (although not the only) hepatotrophic molecule in portal blood; that insulin is avidly removed by the liver; and that its primary passage through the hepatic microvasculature is crucial for the maintenance of liver size, ultrastructure, function, and the capacity for regeneration (27,28,116–122). When other molecules subsequently were identified that had insulin-like or diametrically opposite effects (Table 4), hepatotrophic physiology blossomed into multiple research areas of metabolism and regenerative medicine (123,124).
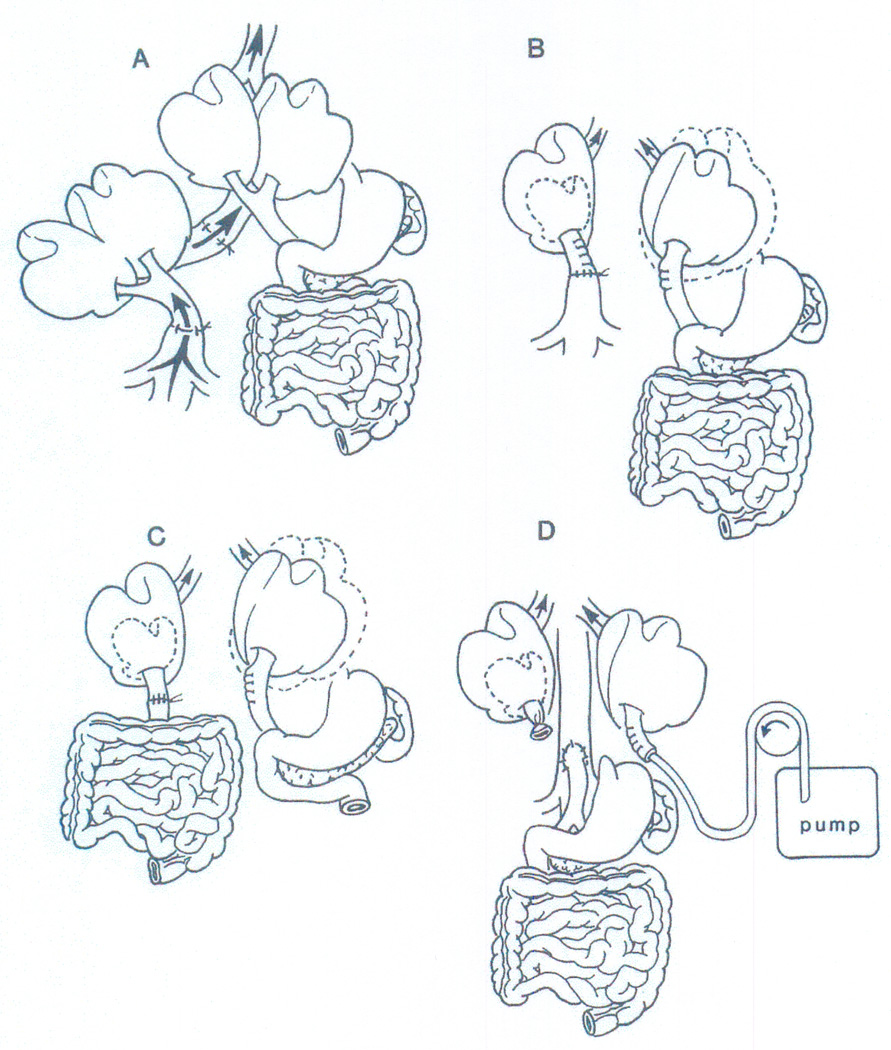
Figure 4.
The double liver models that led to progressively precise identification of the hepatotrophic factors that influenced liver size, ultrastructure, function, and the capacity for regeneration: (A) Welch’s index operation of auxiliary liver allotransplantation (see also Figure 1); (B) non-transplant split liver model that differentiated the effect on the liver of systemic venous (vena caval) versus splanchnic (portal) blood; (C) separation with the double liver fragment model of the qualities of venous blood from the upper and lower abdominal viscera; and (D) selective infusion of candidate hepatotrophic molecules into one or the other of 2 liver fragments, both of which had an arterial supply only. From: Liver Transplant & Surg 4:1-14, 1998.
TABLE 4.
HEPATOTROPHIC FACTORS REVEALED BY 1994 WITH PORTAL DIVERSION, DOUBLE LIVER FRAGMENT, OR PARTIAL HEPATECTOMY MODELS* Annotated in Hepatology 20:747-757, 1994 (Ref 124)
| Hepatotrophic | |
|---|---|
| Hormones: | |
| Insulin | |
| Growth factors: | |
| Cytosol substrate and ALR | |
| IGF II | |
| TGF-α a | |
| HGF a | |
| Immunosuppressants: | |
| Cyclosporine | |
| Tacrolimus | |
| Immunophilins: | |
| FKBP12 | |
| Anti-hepatotrophic | |
| Growth factors: | |
| TGFβb | |
| Immunosuppression: | |
| Rapamycinb | |
It is noteworthy that numerous humeral and cellular mechanisms involved in liver size homeostasis and regeneration (not shown here) are the same as those involved in immunologic responsiveness (rejection) and unresponsiveness (tolerance).
Mitogenic in tissue culture
Inhibitory in tissue culture
Although the moratorium studies did not support reconsideration of auxiliary liver transplant trials, they added a new dimension to the operation of portacaval shunt which had been used primarily to treat complications of portal hypertension. With the demonstration of the profound effects of portal diversion on protein, carbohydrate (119), and lipid metabolism (121), portacaval shunt was used to favorably alter the course of 3 categories of inheritable metabolic disorders: glycogen storage diseases (125,126), familial hyperlipoproteinemia (127,128), and alpha-1-antitrypsin deficiency (129,130). The dramatic amelioration of the pathophysiology of these diverse conditions (e.g. hyperlipoproteinemia, Figure 5) presaged their definitive correction with liver replacement (see next section).
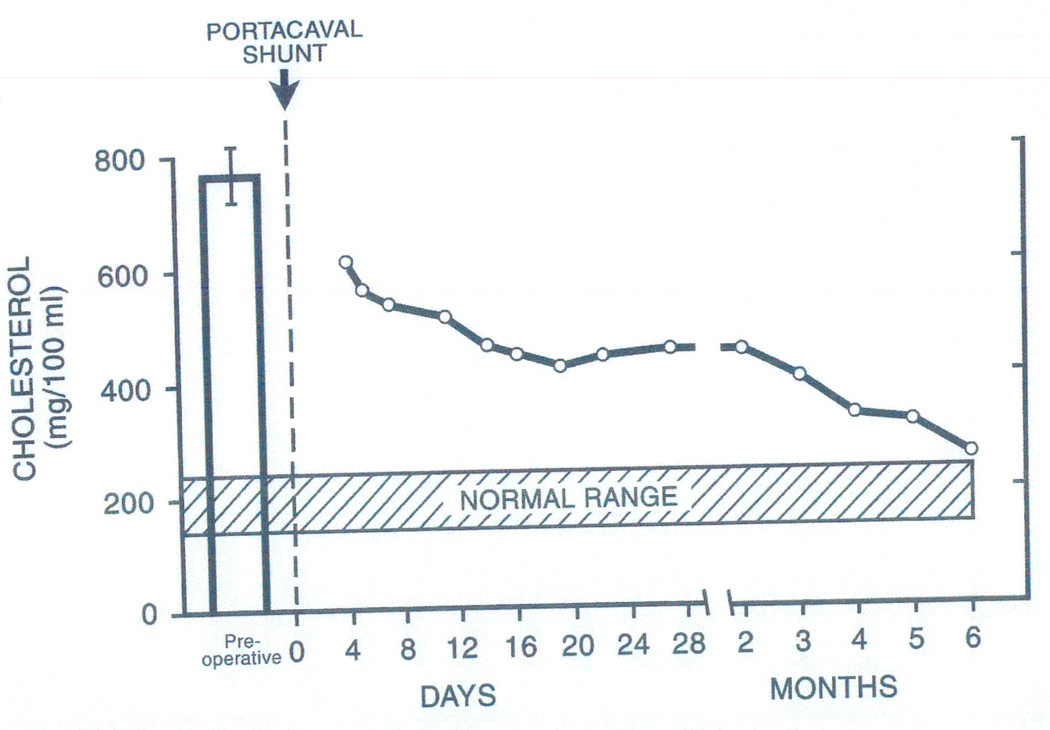
Figure 5.
The dramatic effect of portacaval shunt on serum cholesterol concentration in a child with homozygous familial hyperlipoproteinemia. These observations (24) and canine studies of lipid synthesis with the models shown in Figure 4 (25) suggested that the liver was the principal site of cholesterol homeostasis. Although we considered familial hyperlipoproteinemia to be a candidate disease for liver replacement from the mid 1970s, this was not accomplished until February 14, 1984 (44,45) by which time more evidence that this was an appropriate step was obtained in New York, Bethesda, and Dallas. Interactions over more than a dozen years between experts in cholesterol metabolism in these cities and the author (TES) are described in the chapter “The Little Drummer Girls” of The Puzzle People (128). From: Lancet 2:940-944, 1973
Themes II (the surgical operations) and III (immunology) were pursued with both kidney and liver canine transplant models. These efforts included the construction and testing of equipment with which livers could be preserved for one or two days (131), the experimental development and clinical introduction of antilymphoid globulin (ALG) (13,132), and the demonstration that immunosuppression-aided organ tolerance was more frequently induced by the liver than by the kidney (12). In addition, studies of our burgeoning human kidney recipient population clarified the role of HLA matching in all kinds of organ transplantation (14).
Activity also had intensified on the humanism issues (Theme V). The agenda items at medical ethics conferences in 1966-67 (15,16) included human experimentation, living organ donation, informed consent, and the equitable allocation of organs. The most definitive consequence of these discussions was an evolving consensus that the end of life was more appropriately defined by brain death than by the previous criteria of cessation of heart beat and respiration (18).
THE LIVER TRANSPLANT BEACHHEAD
Despite these accomplishments, confidence about our impending liver trial was nowhere near the level that had existed during the run up to the 1963 attempts. The legacy of doubt from the earlier failures was cancelled by a critical new factor. This was the arrival in 1966 of Carl Groth, a 32 year old Fulbright Fellow from Stockholm who joined all of the thematic developments and became a key member of both the donor and recipient teams. With Groth’s leadership, multiple examples of prolonged human liver recipient survival were produced in 1967 (Figure 6), using triple drug immunosuppression (azathioprine, prednisone, and ALG) (17).
Figure 6.
The first 3 human recipients with prolonged survival following liver replacement in July and August, 1967. The adult, Carl Groth, was a Swedish surgeon-in-training whose tenures in Denver as a Fulbright Scholar (1966-68) and faculty member (1970-71) were near the beginning of his Olympian career. After returning to Stockholm to occupy a Chair in transplantation surgery created for him at the Karolinska Institute, Groth developed the multiorgan transplant program that produced the first liver transplantations in Sweden. His numerous honors include the King’s Medal of his country and the Medawar Prize, the highest distinction of the international Transplantation Society.
The first Denver successes were bolstered by the opening in 1968 of a second clinical liver program by Roy Calne in Cambridge, England (133), following preclinical studies in outbred pigs (21,134). The early trials were described in my 1969 book entitled, Experience in Hepatic Transplantation (22), based on our first 25 human liver replacements and 8 performed elsewhere (4 by Calne). Collateral support was provided with the use of the same immunosuppression regimen for the first successful human heart, lung, and pancreas transplantations (135–137) (Table 5). However, the promise of the non-renal procedures, and even of deceased donor kidney transplantation, was unfulfilled for the next dozen years because of immunosuppression-related morbidity and mortality.
TABLE 5.
THE DOMINO EFFECT IN 1968-69 OF THE 1967 FIRST SUCCESSFUL HUMAN LIVER TRANSPLANTATIONS
| ORGAN | CITY | DATE | PHYSICIAN/ SURGEON |
REF |
|---|---|---|---|---|
| Kidney | Boston | 1/24/59 | Merrill/Murray | 91 |
| Liver | Denver | 7/23/67 | Starzl | 17 |
| Heart | Cape Town | 1/2/68 | Barnard | 135 |
| Lung* | Ghent | 11/14/68 | Derom | 136 |
| Pancreas** | Minneapolis | 6/3/69 | Lillehei | 137 |
Patient died after 10 months; all others in table lived >one year with functioning graft. The first >one year survival of isolated lung recipients was not reported until 1987.
Kidney and pancreas allografts in a uremic patient.
Half or more of the liver recipients treated during this time died within the first post-transplant year. The most encouraging observation was that many patients who survived to this milestone were quietly compiling years of good health thereafter (64,155) (Figure 7). Despite deepening suspicion that progress in the whole field of organ transplantation had permanently stalled, the new French and German liver teams of Henri Bismuth and Rudolf Pichlmayr joined the Denver-Cambridge (Eng) alliance in the early 1970s, followed later in the decade by the Dutch group of Rudi Krom. Much of the medical-scientific, logistic, and administrative framework of hepatic transplantation that exists today was developed by the 5 mutually supportive liver centers during the frustrating period between 1969 and 1979.
Figure 7.
World’s longest surviving liver recipient whose 40th post-transplant anniversary will take place January 22, 2010. The primary disease diagnosis was biliary atresia, but the right lobe of her excised liver contained an incidental 2.7 × 1.8 centimeter hepatoma. The serum alpha fetoprotein level was 6 mg/cm at one post-transplant month, trace-present at 4 months, and undetectable since (Ref 189). The patient’s companion, now a retired United States Marine, is her husband of many years. The statue behind them is Roberto Clemente (1934–1972), the greatest baseball right fielder of all time who was killed bringing food by air flight to victims of the catastrophic Nicaraguan earthquake.
Most of the indications for liver transplant candidacy were obvious, including inheritable disorders with a definitive biochemical explanation (e.g. Wilson’s disease [23]). The acid test of liver transplantation ultimately would help elucidate the mechanisms or pathophysiology of less well-understood inborn errors: e.g. the 3 diseases that were palliated by portacaval shunt (see earlier). Four patients with alpha-1-antitrypsin deficiency underwent liver transplantation between 1973–1977 (138,139). Liver replacement for treatment of glycogen storage disorders (140,141), hyperlipoproteinemia (44,45), and a growing panoply of other metabolic diseases awaited better immunosuppression.
THE LIVER AVALANCHE
Improvements in therapy were heralded in 1979 by Roy Calne’s report of cyclosporine-based immunosuppression in 34 patients, including two liver recipients (33). The side effects of cyclosporine precluded its use as a single agent. However, when it was substituted for azathioprine in our two- or three-drug therapeutic algorithm that included dose-maneuverable prednisone (34), cyclosporine’s full potential was realized. Kidney recipients were the first to be treated with liver recipients close behind. Eleven of our first 12 liver recipients treated in Colorado with cyclosporine-based immunosuppression during 1979-80 survived for more than one year (35).
More experience in 1981-82 (now in Pittsburgh) was confirmatory. In December 1981, these findings were reported to C. Everett Koop, the United States Surgeon General, who initiated a Consensus Development Conference for liver transplantation that would include input from the European centers. Prior to the Conference, I prepared a summary of our experience for presentation on November 1, 1982, at the American Association for the Study of Liver Disease (AASLD), and publication in Hepatology the same month (36). An updated version was presented to the Consensus Development Conference on June 20–23, 1983.
The consensus committee concluded that liver transplantation had become a “clinical service” as opposed to an experimental procedure (38). The resulting world-wide stampede to develop liver transplant centers was even more dramatic than that of kidney transplantation 20 years earlier. Only 6 years after the Consensus Conference, a 17 page article equally divided between the October 12 and October 19 issues of the New England Journal of Medicine (142) contained a opening statement that, “The conceptual appeal of liver transplantation is so great that the procedure may come to mind as a last resort for virtually every patient with lethal hepatic disease.” It already was evident that the need for these operations would greatly exceed both an identifiable source of organs and those qualified to transplant them.
A significant number of the next generation of liver transplant leaders who flocked to Pittsburgh for clinical training during the 1980s were non-surgeons. Their primary connection was with David Van Thiel (Figure 8), the brilliant gastroenterologist who became a founding doyen of transplantation hepatology along with his English counterpart, Roger Williams of the Cambridge-King’s College program. During this volatile period, preclinical studies of tacrolimus were begun that would lead to its substitution for cyclosporine (56,57) with fast-track FDA approval in November1993. With tacrolimus, the multivisceral and intestine-alone transplant procedures developed 3 decades earlier in dogs (Figure 3) achieved the status of a genuine “clinical service” (61,62). The timing was perfect. With arrival of my 65th birthday in 1991, I retired from active surgical practice.
Figure 8.
David Van Thiel (1941-), gastroenterologist-hepatologist without whose herculean efforts, the University of Pittsburgh liver transplantation program could not have been established.
THEMATIC EPILOGUE: 1991 – 2009
Most of the advances in liver transplantation during the succeeding 18 years (Table 1) have been derivative from earlier work including the use of partial livers from deceased or living volunteer donors. However, the antecedent contributions with which the taxonomical foundation of organ transplantation was built have been obscured with the advent of the World Wide Web (www). Many of the referenced articles of the foregoing narrative cannot be accessed online in full text, and some have become invisible. With the dearth of electronic information from before the 1990s and the convenience of citing easy internet finds, the recent literature has been replete with observations, events, and concepts that were described more clearly years or decades before. Nevertheless, there have been new trends in organ transplantation, 2 of which were driven mainly by the liver.
The Exegesis Of Alloengraftment
A major gap in immunology (Theme III) when I stopped surgical practice was the inability to explain why organ transplantation had been possible. Because organ recipients were not infused with donor leukocytes, it became dogma by the early 1960s that the donor leukocyte chimerism associated with acquired tolerance in experimental models was not a factor in organ engraftment. The dogma was not challenged until we discovered small numbers of multilineage donor leukocytes (microchimerism) in the blood or tissues of all studied long-surviving liver, kidney, and other organ recipients (63,64,143). These findings in 1992-93, and an array of supporting experimental studies in congenic rat (144–150) and mouse models (151–154) mandated a change in the previously perceived landscape of transplantation immunology.
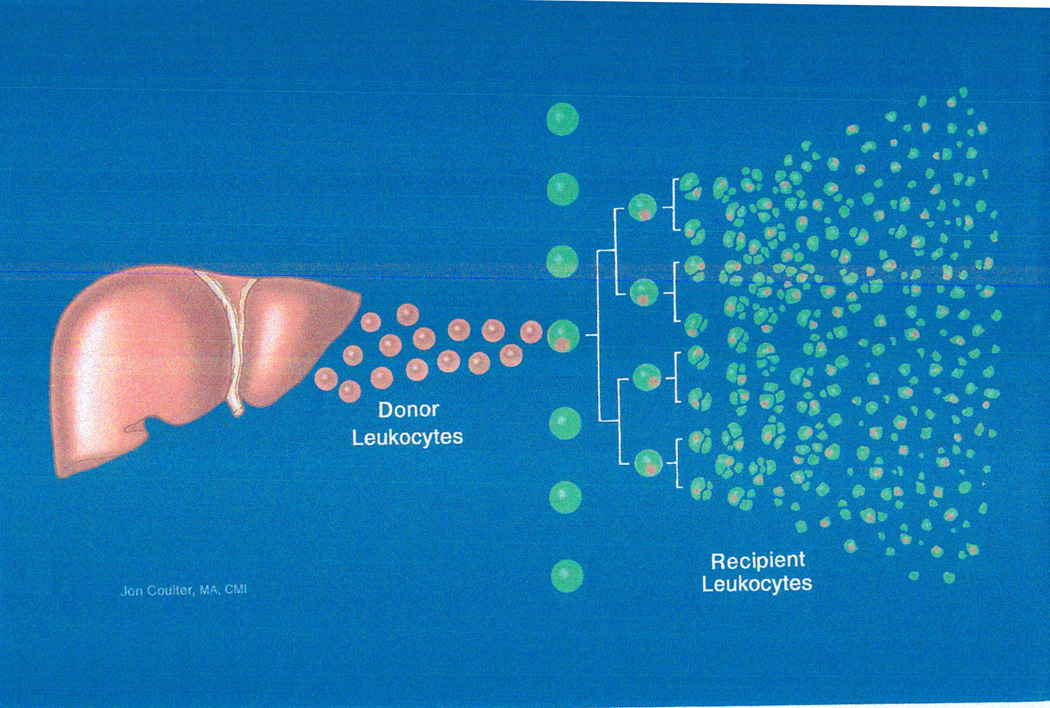
It was proposed (63,64,155,156) that organ transplantation was the equivalent of a bone marrow transplantation. The key step leading to rejection, or alternatively alloengraftment, after both kinds of transplantation was hematogenous migration of leukocytes (including stem cells [157–159]) to the recipient’s lymphoid organs (Figure 9). Otherwise, the presence of the allograft would not be recognized: i.e. the “immune ignorance” (160,161) first described in a transplant model by Clyde Barker and Rupert Billingham 42 years ago. The seminal mechanism of alloengraftment was exhaustion-deletion of the T cell response (162,163) induced at the host lymphoid sites by the invading cells (Figure 9). Because the migrant donor leukocytes are immune competent, successful alloengraftment involved a double immune reaction in which immune responses of coexisting donor and recipient cells, each to the other, were reciprocally exhausted and deleted under a protective umbrella of immunosuppression (Figure 10).
Figure 9.
The cell migration and localization of organ and bone marrow cell transplantation. Organs (here a liver) are composites of architecturally fixed cells and mobile multilineage cells of bone marrow origin (“passenger leukocytes”) that include pluripotent hematolymphopoietic stem cells (157-159). Within minutes after organ transplantation, the passenger leukocytes simulate a bone marrow cell infusion by migrating selectively to recipient lymphoid organs where they induce the depleted antidonor T cell response. Although the clonal response normally destroys the invading donor cells and their outlying source organ (rejection), the response may be exhausted and deleted if it is too weak to eliminate the invading donor cells during the first few weeks of maximal cell migration. Perpetuation thereafter of survival of the bystander organ allograft requires persistence of enough donor leukocytes to maintain the initial exhaustion-deletion. Importantly, the invading donor cells are immune competent and their response against the recipient also must be exhausted and deleted for a successful transplant outcome (see Figure 10).
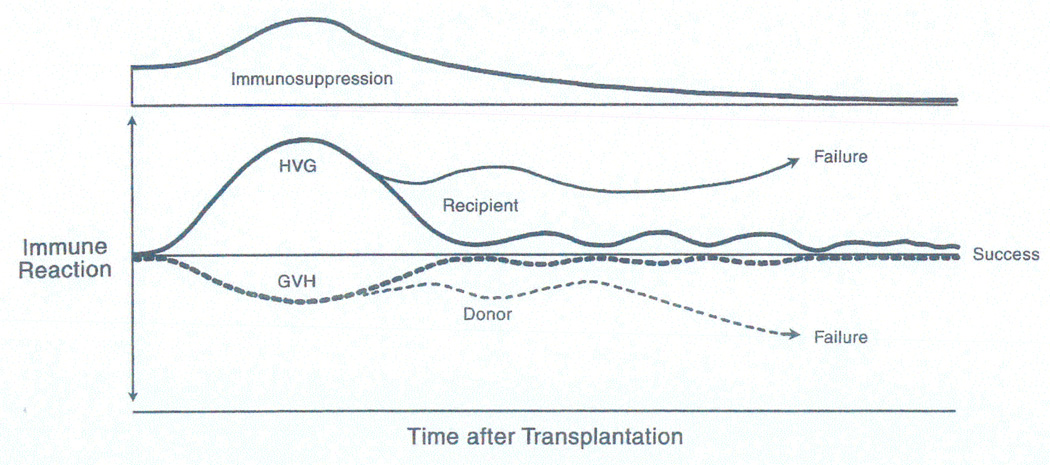
Figure 10.
The kinetics of immunosuppression-aided exhaustion and deletion of the contemporaneous host versus graft (HVG, upright curve) and graft versus host (GVH, inverted curve) responses in organ recipients following the cell migration shown in Figure 8. Although HVG is the dominant response in most organ recipients (expressed as rejection), serious or lethal GVH reactions (expressed as graft versus host disease [GVHD]) are not rare in recipients of lymphoid-rich organs (liver, intestine). In naturally immune deficient or cytoablated bone marrow cell recipients, GVHD is avoided by using histocompatible (HLA-matched) donors. Therapeutic failure after either organ or bone marrow cell transplantation implies the inability to control one, the other, or both of the responses. From New Engl J Med 339:1905-1913, 1998.
Our interpretation of the microchimerism was at first highly controversial (164,165) because it was incompatible with multiple theories and hypothesis that made up much of the base of transplant immunology. Resistance to the new concept was eroded when Rolf Zinkernagel in Zurich independently proposed an explanation of acquired tolerance to pathogens that was essentially the same as that of our allotolerance paradigm. In the 1970’s, Zinkernagel and Doherty had demonstrated that the MHC-restricted cytolytic T cell response induced by noncytopathic microorganisms was the same as that induced by allografts. These studies were done in highly controlled experimental models of infection with the lymphocytic choriomeningitis virus (LCMV) and other intracellular parasites (166). Their subsequent investigations of tolerance were done with the same models and described in 4 landmark articles between 1993 and 1997 (167–170).
With recognition that the Pittsburgh and Zurich investigations were on parallel pathways, a joint author review was published in a December 1998 issue of the New England Journal of Medicine in which analogous scenarios were described of transplantation and pathogen-specific infections (e.g. chronic rejection vis a vis chronic viral hepatitis) (65). The concept developed from transplant and infection models was generalized in the following way: “The migration and localization of antigen govern the immunologic responsiveness or unresponsiveness against infections, tumors, or self --- and against xenografts or allografts” (65). In this view, all outcomes in the divergent circumstances of transplantation including those of microchimerism (150,171,172) were determined by the balance established between the amount of mobile donor leukocytes with access to host lymphoid organs and the number of donor-specific cytolytic T-cells (CTL) induced at the lymphoid sites (Figure 11, inner graphic) (65).
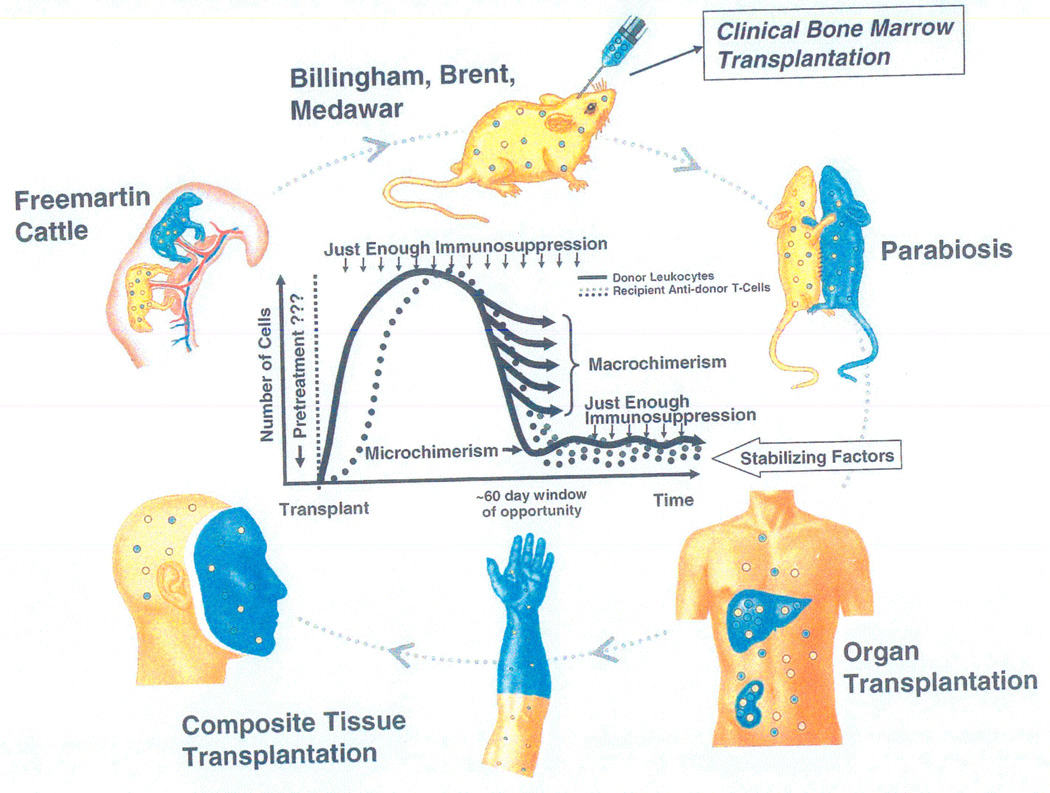
Figure 11.
The many faces of transplantation tolerance.
Outer Circle: The continuum of experimental and clinical donor leukocyte chimerism-associated tolerance models that can be traced back to observations in 1945 in freemartin cattle (upper left) whose fused placentas permitted fetal cross-circulation, blood chimerism, and reciprocal immune nonreactivity.
Inner Graphic: Permutations of tolerance defined as balances between persisting migratory donor leukocytes and the number of antidonor T cells undergoing steady state exhaustion-deletion. The achievement of balances and the resulting clinical phenotypes are influenced by the dose, type, and timing of immunosuppressive therapy and by the dose, type, timing, route, and localization of the migrant donor cells. The single most important factor leading to the macrochimerism of bone marrow cell transplantation versus the microchimerism of most organ (and composite tissue) recipients is enfeeblement of recipient immune reactivity before the arrival of donor cells in the first instance and after their arrival in the second. The non-specific potential “stabilizing factors” in the left-directed arrow above the human silhouette include special cells (e.g. T-regulatory), enhancing antibodies, graft secretions, and endogenous cytoprotective molecules.
Long term organ alloengraftment with this generalizable paradigm, was a highly variable form of leukocyte chimerism-dependent tolerance, the completeness of which could be inferred from the amount of immunosuppression necessary to maintain stable function and structure of the transplant (Figure 11). In a second article with Zinkernagel, the Pittsburgh-Zurich immunologic paradigm provided a road map for improved therapeutic strategies of transplant patient management based on 2 principles: recipient pretreatment, and the least possible use of post-transplant immunosuppression (68). When applied clinically for different kinds of organ transplantation (69), these strategies have minimized, or in some cases eliminated, the burden of chronic immunosuppression (173–178). More rational approaches also were developed for the treatment of opportunistic infections caused by noncytopathic microorganisms (70,168,179).
Reporting of Transplantation Outcomes (Theme IV) and Equitable Organ Allocations (Theme V)
A second trend coincided with and was empowered by the rise of the internet. One of the mandates of the 1984 National Transplant Act was the formation of an organ procurement and transplantation network (OPTN). Another was the development of a scientific registry of transplant recipients (SRTR) with which patient and graft survival could be quantified from center to center along with center-specific parameters. After the Department of Health Resources and Services Administration (DHHS) awarded the contract for both functions to the United Network of Organ Sharing (UNOS), disputes about organ allocation within the appointed UNOS committee prevented the development of the required plan. In order to avoid a UNOS default of contract, a document was pieced together from 2 articles In Press describing the renal (180) and non-renal (181) distribution systems already in place in Pittsburgh.
In the contract derived from these manuscripts and presented to DHHS on the eve of the deadline, the overwhelming factor for liver distribution was recipient urgency of need (181). In contrast, time waiting dominated kidney distribution with major credit for HLA matching only when this was complete (180). Although these policies were accepted by DHHS and provisionally implemented in November 1987, they were widely abridged (182) until the final regulations were issued by DHHS on April 2, 1998. During the chaotic intervening decade (see Supplementary Index for a cryptic description of the “liver wars”), UNOS led the opposition to adoption of the regulations and withheld access to SRTR. A Lancet Editorial during the heat of the debates suggested that: “UNOS would better serve the transplant community if it abandoned its stance and began working with DHHS to draw up allocation policies that are practical and fair (183)”.
One of the most contentious issues was the conclusion in a large Pittsburgh study published in 1994 that liver transplantation performed too early was associated with a net loss of recipient life years (184,185). These findings led to retention of the “sickest first” policy in both the provisional and final DHHS rules for liver allocation. In the meanwhile, the continued resistance to release of center-specific data, as well as inaccuracies and inconsistencies in the first SRTR reports (1992, 1995, 1997), led to transfer of SRTR management to the University of Michigan-based Arbor Research Collaborative for Health. An Arbor multicenter study in 2005 confirmed the original Pittsburgh findings about the timing of liver transplantation and came to the same policy recommendations (186).
Until now, success with liver transplantation has been judged largely by relatively short term patient and graft survival. A more complete profile has been made possible by the use of the treatment-based evaluation system of Clavien in which the rate and severity of complications (including death) are quantified with a 5-tier scale (71). The value of this objective assessment was exemplified by a recent Pittsburgh study of right lobar living donor liver transplantation (187). The Clavien metric is applicable to all kinds of organ transplantation, and has been generalized to other surgical and medical procedures (188).
CONCLUSION
Liver transplantation began with almost no resources at the same time as the tentative first steps were taken to land a man on the moon. Because human lives would be at stake, both objectives had a sacramental element from the outset: i.e. a solemnly binding commitment to perfection. A need for that pledge still exists.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ms. Terry L. Mangan for her assistance in manuscript preparation. We also thank Mr. Ed Gray, a Systems Engineer, for his honest broker and intellectual contributions between 1999–2009 without which this manuscript could not have been written.
This work was supported by National Institutes of Health Grant DK29961, and unrestricted gifts from Frank and Athena Sarris and Mr. Robert Eberly.
REFERENCES
- 1.Welch CS. A note on transplantation of the whole liver in dogs. Transplant Bull. 1955;2:54–55. [Google Scholar]
- 2.Starzl TE, Bernhard VM, Benvenuto R, Cortes N. A new method for one-stage hepatectomy in dogs. Surgery. 1959;46:880–886. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Kaupp HA, Jr, Brock DR, Lazarus RE, Johnson RV. Reconstructive problems in canine liver homotransplantation with special reference to the postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733–743. [PMC free article] [PubMed] [Google Scholar]
- 4.Moore FD, Smith LL, Burnap TK, Dallenbach FD, Dammin GJ, Gruber UF, Shoemaker WC, Steenburg RW, Ball MR, Belko JS. One-stage homotransplantation of the liver following total hepatectomy in dogs. Transplant Bull. 1959;6:103–110. doi: 10.1097/00006534-195901000-00041. [DOI] [PubMed] [Google Scholar]
- 5.Moore FD, Wheeler HB, Demissianos HV, Smith LL, Balankura O, Abel K, Greenberg JB, Dammin GJ. Experimental whole organ transplantation of the liver and of the spleen. Ann Surg. see also discussion of Moore’s presentation by Starzl TE. 1960;1960;152152:374–385. 386. [PMC free article] [PubMed] [Google Scholar]
- 6.Starzl TE, Kaupp HA., Jr Mass homotransplantation of abdominal organs in dogs. Surg Forum. 1960;11:28–30. [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–229. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 9.Marchioro TL, Huntley RT, Waddell WR, Starzl TE. Extracorporeal perfusion for obtaining postmortem homografts. Surgery. 1963;54:900–911. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Marchioro TL, Von Kaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 11.Starzl TE, Marchioro TL, Rowlands DT, Jr, Kirkpatrick CH, Wilson WEC, Rifkind D, Waddell WR. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann Surg. 1964;160:411–439. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Marchioro TL, Porter KA, Taylor PD, Faris TD, Herrmann TJ, Hlad CJ, Waddell WR. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Marchioro TL, Porter KA, Iwasaki Y, Cerilli GJ. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Porter KA, Andres G, Halgrimson CG, Hurwitz R, Giles G, Terasaki PI, Penn I, Schroter GT, Lilly J, Starkie SJ, Putnam CW. Long-term survival after renal transplantation in humans: With special reference to histocompatibility matching, thymectomy, homograft glomerulonephritis, heterologous ALG, and recipient malignancy. Ann Surg. 1970;172:437–472. doi: 10.1097/00000658-197009000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolstenholme GEW, O’Connor M, editors. Ethics in Medical Progress: With Special Reference to Transplantation. Boston: Little Brown and Co; 1966. pp. 1–249. [Google Scholar]
- 16.Elkinton JR, editor. The Changing Mores of Biomedical Research: A Colloquim on Ethical Dilemmas from the Medical Advances. Annals of Internal Medicine. 1967;67(supp 17):1–77. The Keynote speakers were: Peter Medawar, Warren Burger, Esq (US Supreme Court), Joshua Lederberg and Thomas Starzl. [PubMed] [Google Scholar]
- 17.Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Jr, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Definition of irreversible coma: Report of the ad hoc committee of the Harvard Medical School to examine the definition of brain death. JAMA. 1968;205:337. [PubMed] [Google Scholar]
- 19.Cordier G, Garnier H, Clot JP, Camplez P, Gorin JP, Clot Ph, Rassinier JP, Nizza M, Levy R. La greffe de foie orthotopique chez le porc. Mem Acad Chir (Paris) 1966;92:799–807. [PubMed] [Google Scholar]
- 20.Peacock JH, Terblanche J. Orthotopic homotransplantation of the liver in the pig. In: Read AE, editor. The Liver. London: Butterworth; 1967. p. 333. [Google Scholar]
- 21.Calne RY, White HJO, Yoffa DE, Binns RM, Maginn RR, Herbertson RM, Millard PR, Molina VP, Davis DR. Prolonged survival of liver transplants in the pig. Br Med J. 1967;4:645–648. doi: 10.1136/bmj.4.5580.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starzl TE. Experience in Hepatic Transplantation WB Saunders Company. Philadelphia, PA: pp. 1969pp. 1–533. [Google Scholar]
- 23.DuBois RS, Giles G, Rodgerson DO, Lilly J, Martineau G, Halgrimson CG, Schroter G, Starzl TE, Sternlieb I, Scheinberg IH. Orthotopic liver transplantation for Wilson’s disease. Lancet. 1971;1:505–508. doi: 10.1016/s0140-6736(71)91121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE, Chase HP, Putnam CW, Porter KA. Portacaval shunt in hyperlipoproteinemia. Lancet. 1973;2:940–944. doi: 10.1016/s0140-6736(73)92599-3. [DOI] [PubMed] [Google Scholar]
- 25.Starzl TE, Lee IY, Porter KA, Putnam CW. The influence of portal blood upon lipid metabolism in normal and diabetic dogs and baboons. Surg Gynecol Obstet. 1975;140:381–396. [PMC free article] [PubMed] [Google Scholar]
- 26.Starzl TE, Ishikawa M, Putnam CW, Porter KA, Picache R, Husberg BS, Halgrimson CG, Schroter G. Progress in and deterrents to orthotopic liver transplantation, with special reference to survival, resistance to hyperacute rejection, and biliary duct reconstruction. Transplant Proc. 1974;6:129–139. [PMC free article] [PubMed] [Google Scholar]
- 27.Starzl TE, Porter KA, Putnam CW. Intraportal insulin protects from the liver injury of portacaval shunt in dogs. Lancet. 1975;2:1241–1246. doi: 10.1016/s0140-6736(75)92076-0. [DOI] [PubMed] [Google Scholar]
- 28.Starzl TE, Watanabe K, Porter KA, Putnam CW. Effects of insulin, glucagon, and insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1(7964):821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 29.Starzl TE, Porter KA, Putnam CW, Schroter GPJ, Halgrimson CG, Weil R, III, Hoelscher M, Reid HAS. Orthotopic liver transplantation in ninety-three patients. Surg Gynecol Obstet. 1976;142:487–505. [PMC free article] [PubMed] [Google Scholar]
- 30.Wall WJ, Calne RY, Herbertson BM, Baker PG, Smith DP, Underwood J, Kostakis A, Williams R. Simple hyptothermic preservation for transporting human livers long distance for homotransplantation. Transplantation. 1977;23:210–216. doi: 10.1097/00007890-197703000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Benichou J, Halgrimson CG, Weil R, III, Koep LJ, Starzl TE. Canine and human liver preservation for 6 to 18 hour by cold infusion. Transplantation. 1977;24:407–411. doi: 10.1097/00007890-197712000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starzl TE, Halgrimson CG, Koep LJ, Weil R, III, Taylor PD. Vascular homografts from cadaveric organ donors. Surg Gynecol Obstet. 1979;149:737. [PMC free article] [PubMed] [Google Scholar]
- 33.Calne RY, Rolles K, White DJ, Thiru S, Evans DB, McMaster P, Dunn DC, Craddock GN, Henderson RG, Aziz S, Lewis P. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 34.Starzl TE, Weil R, III, Iwatsuki S, Klintmalm G, Schroter GPJ, Koep LJ, Iwaki Y, Terasaki PI, Porter KA. The use of cyclosporin A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 35.Starzl TE, Klintmalm GBG, Porter KA, Iwatsuki S, Schroter GPJ. Liver transplantation with use of cyclosporin A and prednisone. N Engl J Med. 1981;305:266–269. doi: 10.1056/NEJM198107303050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, Iwatsuki S, Van Thiel DH, Gartner JC, Zitelli BJ, Malatack JJ, Schade RR, Shaw BW, Jr, Hakala TR, Rosenthal JT, Porter KA. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw BW, Jr, Martin DJ, Marquez JM, Kang YG, Bugbee AC, Iwatsuki S, Griffith BP, Hardesty RL, Bahnson HT, Starzl TE. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–534. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Institutes of Health Consensus Development Conference on Liver Transplantation. Sponsored by the National Institute of Arthritis, Diabetes, and Digestive and Kidney Diseases and the National Institutes of Health Office of Medical Applications of Research. Hepatology. 1983;4(Suppl 1):1S–110S. 1984. [PubMed] [Google Scholar]
- 39.Starzl TE, Hakala TR, Shaw BW, Jr, Hardesty RL, Rosenthal TJ, Griffith BP, Iwatsuki S, Bahnson HT. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–230. [PMC free article] [PubMed] [Google Scholar]
- 40.Starzl TE, Miller C, Broznick B, Makowka L. An improved technique for multiple organ harvesting. Surg Gynecol Obstet. 1987;165:343–348. [PMC free article] [PubMed] [Google Scholar]
- 41.Starzl TE, Nalesnik MA, Porter KA, Ho M, Iwatsuki S, Griffith BP, Rosenthal JT, Hakala TR, Shaw BW, Jr, Hardesty RL, Atchison RW, Jaffe R, Bahnson HT. Reversibility of lymphomas and lymphoproliferative lesions developing under cyclosporin-steroid therapy. Lancet. 1984;1:583–587. doi: 10.1016/s0140-6736(84)90994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367–370. [PubMed] [Google Scholar]
- 43.Broelsch CE, Neuhaus P, Burdelski M, et al. Orthotope transplantation von Lebegmenten bei mit Gallengangsatresien. (Orthotopic transplantation of hepatic segments in infants with biliary atresia) In: Kolsowski L, editor. Chirurgisches Forum 1984, F Experim U Klimische Forschung Hrsga. Berline: Springer-Verlag; 1984. pp. 105–109. [Google Scholar]
- 44.Starzl TE, Bilheimer DW, Bahnson HT, Shaw BW, Jr, Hardesty RL, Griffith BP, Iwatsuki S, Zitelli BJ, Gartner JC, Jr, Malatack JJ, Urbach AH. Heart-liver transplantation in a patient with familial hypercholesterolemia. Lancet. 1984;1:1382–1383. doi: 10.1016/s0140-6736(84)91876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bilheimer DW, Goldstein JL, Grundy SC, Starzl TE, Brown MS. Liver transplantation provides low-density-lipoprotein receptors and lowers plasma cholesterol in a child with homozygous familial hypercholesterolemia. N Engl J Med. 1984;311:1658–1664. doi: 10.1056/NEJM198412273112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starzl TE, Rowe MI, Todo S, Jaffe R, Tzakis A, Hoffman AL, Esquivel C, Porter KA, Venkataramanan R, Makowka L, Duquesnoy R. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 47.Grant D, Wall W, Mimeault R, Zhong R, Ghent C, Garcia B, Stiller C, Duff J. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–184. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 48.Jamieson NV, Sundberg R, Lindell S, Laravuso M, Kalayoglu M, Southard JH, Belzer FO. Successful 24- to 30-hour preservation of the canine liver: a preliminary report. Transplant Proc. 1988;20(Suppl 1):945–947. [Google Scholar]
- 49.Kalayoglu M, Sollinger HW, Stratta RJ, D’Alessandro AM, Hoffmann RM, Pirsch JD, Belzer FO. Extended preservation of the liver for clinical transplantation. Lancet. 1988;1:617–619. doi: 10.1016/s0140-6736(88)91416-x. [DOI] [PubMed] [Google Scholar]
- 50.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989;261:711–714. [PMC free article] [PubMed] [Google Scholar]
- 51.Makowka L, Gordon RD, Todo S, Ohkohchi N, Marsh JW, Tzakis AG, Yokoi H, Ligush J, Esquivel CO, Satake M, Iwatsuki S, Starzl TE. Analysis of donor criteria for the prediction of outcome in clinical liver transplantation. Transplant Proc. 1987;19:2378–2382. [PMC free article] [PubMed] [Google Scholar]
- 52.Starzl TE, Hakala T, Tzakis A, Gordon R, Stieber A, Makowka L, Klimoski J, Bahnson HT. A multifactorial system for equitable selection of cadaveric kidney recipients. JAMA. 1987;257:3073–3075. [PMC free article] [PubMed] [Google Scholar]
- 53.Starzl TE, Gordon RD, Tzakis A, Staschak S, Fioravanti V, Broznick B, Makowka L, Bahnson HT. Equitable allocation of extrarenal organs: With special reference to the liver. Transplant Proc. 1988;20:131–138. [PMC free article] [PubMed] [Google Scholar]
- 54.Tzakis A, Todo S, Starzl TE. Orthotopic liver transplantation with preservation of the inferior vena cava. Ann Surg. 1989;210:649–652. doi: 10.1097/00000658-198911000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Starzl TE, Todo S, Tzakis A, Podesta L, Mieles L, Demetris A, Teperman L, Selby R, Stevenson W, Stieber A, Gordon R, Iwatsuki S. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann Surg. 1989;210:374–386. doi: 10.1097/00000658-198909000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Todo S, Fung JJ, Starzl TE, Tzakis A, Demetris AJ, Kormos R, Jain A, Alessiani M, Takaya S. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pichlmayr R, Ringe B, Gubernatis G, et al. Transplantation einer spenderleber auf Zwis Empfanger (Split liver transplantation) Eine neue methode in der weitzentwicklung der lebesegment transplantation. Langenbecks Archiv Surg. 1989;373:127–130. [PubMed] [Google Scholar]
- 59.Strong RW, Lynch SV, Ong TH, Matsunami H, Koido Y, Balderson GA. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322:1505–1507. doi: 10.1056/NEJM199005243222106. [DOI] [PubMed] [Google Scholar]
- 60.Broelsch CE, Emond JC, Whitington PF, Thistlethwaite JR, Baker AL, Lichtor JL. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368–375. doi: 10.1097/00000658-199009000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Todo S, Tzakis AG, Abu-Elmagd K, Reyes J, Nakamura K, Casavilla A, Selby R, Nour BM, Wright H, Fung JJ, Demetris AJ, Van Thiel DH, Starzl TE. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223–234. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Todo S, Tzakis AG, Abu-Elmagd K, Reyes J, Fung JJ, Casavilla A, Nakamura K, Yagihashi A, Jain A, Murase N, Iwaki Y, Demetris AJ, Van Thiel D, Starzl TE. Cadaveric small bowel and small bowel-liver transplantation in humans. Transplantation. 1992;53:369–376. doi: 10.1097/00007890-199202010-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, Ramos H, Todo S, Tzakis A, Fung JJ, Nalesnik M, Zeevi A, Rudert WA, Kocova M. Cell migration and chimerism after whole-organ transplantation: The basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 65.Starzl TE, Zinkernagel R. Antigen localization and migration in immunity and tolerance. New Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamaoka Y, Washida M, Honda K, Tanaka K, Mori K, Shimahara Y, Okamoto S, Ueda M, Hayashi M, Tanaka A, Morimoto T, Ozawa K. Liver transplantation using a right lobe graft from a living related donor. Transplantation. 1994;57:1127–1130. [PubMed] [Google Scholar]
- 67.Wachs M, Bak T, Karrer F, Everson GT, Shrestha R, Trouillot TE, Mandell MS, Steinberg TG, Kam I. Adult Living donor liver transplantation using a right hepatic lobe. Transplantation. 1998;66:1313–1316. doi: 10.1097/00007890-199811270-00008. [DOI] [PubMed] [Google Scholar]
- 68.Starzl TE, Zinkernagel R. Transplantation tolerance from a historical perspective. NATURE Reviews: Immunology. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Starzl TE, Murase N, Abu-Elmagd K, Gray EA, Shapiro R, Eghtesad B, Corry RJ, Jordan ML, Fontes P, Gayowski T, Bond G, Scantlebury VP, Potdar S, Randhawa P, Wu T, Zeevi A, Nalesnik MA, Woodward J, Marcos A, Trucco M, Demetris AJ, Fung JJ. Tolerogenic immunosuppression for organ transplantation. The Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eghtesad B, Fung JJ, Demetris AJ, Murase N, Ness R, Bass DC, Gray EA, Shakil O, Marcos A, Starzl TE. Mechanism-based principles of immunosuppression for liver transplantation in HCV-infected patients. Liver Transplantation. 2005;11:1343–1352. doi: 10.1002/lt.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.