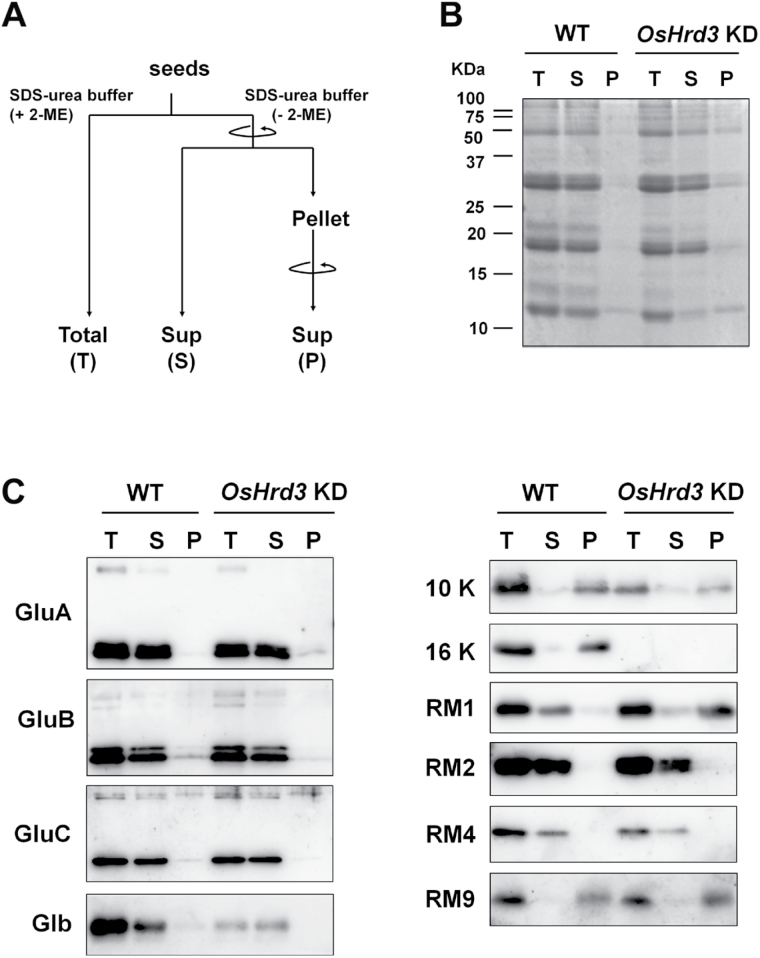
Fig. 4.
Aberrant aggregation of RM1 in OsHrd3 KD seeds. (A) Schematic representation of the experiment. Total proteins were extracted from mature wild-type (WT) and OsHrd3 KD seeds with SDS–urea buffer without 2-mercaptoethanol and fractionated into the supernatant (S) and pellet (P) by centrifugation. The resulting pellets were again extracted with SDS–urea buffer containing 2-mercaptoethanol to collect the solubilized proteins, and proteins in the S fraction were denatured in the presence of 2-mercaptoethanol. For a control, total proteins (T) were extracted from mature WT and OsHrd3 KD seeds with SDS–urea buffer supplemented with 2-mercaptoethanol. (B) SDS–PAGE analysis of the T, S, and P fractions derived from WT and OsHrd3 KD seeds. Total proteins of the T, S, and P fractions were subjected to immunoblot analyses using antibodies against rice seed storage proteins. (C) Immunoblot analysis of the total (T), soluble (S), and pellet (P) fractions derived from WT and OsHrd3 KD seeds. Total proteins of the T, S, and P fractions were subjected to immunoblot analyses using antibodies against rice seed storage proteins.