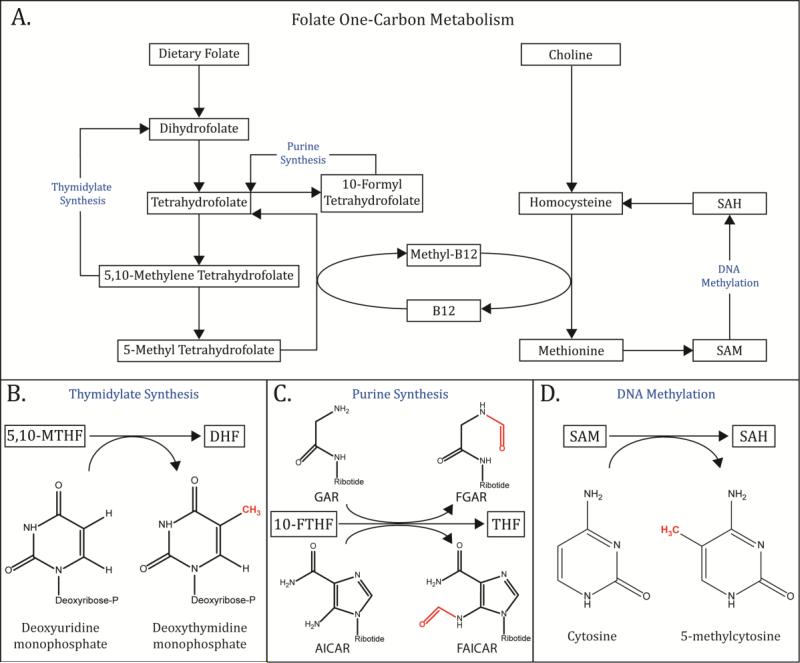
Figure 1.
(A) Overview of folate metabolism. Dietary folate is converted into several key metabolites by a series of enzymatic conversions. (B) 5,10-Methylene Tetahydrofolate is used as the substrate for thymidylate synthesis, where a methyl group is donated to deoxyuridine monophosphate (dUMP) to form deoxythymidine monophosphate (dTMP). (C) 10-Formyl Tetrahydrofolate performs a similar function in the synthesis of the purine nucleotide precursor, inosine monophosphate (IMP). Initially, 10-FTHF donates a formyl group to convert glycinamide ribonucleotide (GAR) to N-formylglycinamide ribonucleotide (FGAR). In a later step, 10-FTHF donates a formyl group to convert 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) into 5-Formamidoimidazole-4-carboxamide ribotide (FAICAR). (D) Methyl groups can be shuttled from 5-Methyl Tetrahydofolate (5-MTHF) to the Homocysteine cycle, where they are used to regenerate the “universal methyl donor”, S-adenosyl methionine (SAM) (Insert III). SAM in turn serves as the substrate for the DNA methyltransferase enzymes, which transfer a methyl group from SAM to cytosine, resulting in the formation of 5-methylcytosine.