Abstract
Although melatonin (MT) has been reported to protect cells against oxidative damage induced by electromagnetic radiation, few reports have addressed whether there are other protective mechanisms. Here, we investigated the effects of MT on extremely low-frequency electromagnetic field (ELF-EMF)-induced Nav activity in rat cerebellar granule cells (GCs). Exposing cerebellar GCs to ELF-EMF for 60 min. significantly increased the Nav current (INa) densities by 62.5%. MT (5 μM) inhibited the ELF-EMF-induced INa increase. This inhibitory effect of MT is mimicked by an MT2 receptor agonist and was eliminated by an MT2 receptor antagonist. The Nav channel steady-state activation curve was significantly shifted towards hyperpolarization by ELF-EMF stimulation but remained unchanged by MT in cerebellar GC that were either exposed or not exposed to ELF-EMF. ELF-EMF exposure significantly increased the intracellular levels of phosphorylated PKA in cerebellar GCs, and both MT and IIK-7 did not reduce the ELF-EMF-induced increase in phosphorylated PKA. The inhibitory effects of MT on ELF-EMF-induced Nav activity was greatly reduced by the calmodulin inhibitor KN93. Calcium imaging showed that MT did not increase the basal intracellular Ca2+ level, but it significantly elevated the intracellular Ca2+ level evoked by the high K+ stimulation in cerebellar GC that were either exposed or not exposed to ELF-EMF. In the presence of ruthenium red, a ryanodine-sensitive receptor blocker, the MT-induced increase in intracellular calcium levels was reduced. Our data show for the first time that MT protects against neuronal INa that result from ELF-EMF exposure through Ca2+ influx-induced Ca2+ release.
Keywords: melatonin, ELF-EMF, Na+ currents, Ca2+ release, cerebellar granule cells
Introduction
Several studies have noted that exposure to extremely low-frequency electromagnetic fields (ELF-EMF) alters animal behaviours and causes biological effects, including changes in gene expression, the regulation of cell survival and the promotion of cell differentiation [1–3]. In addition, exposure to EMF induces changes in cerebral blood flow in old Alzheimer's mice [4]. Enzyme activity in cytosol or at the membrane and subsequent alterations in intracellular signalling are found in lymphoma B cells and Chinese hamster lung cells upon exposure to ELF-EMF [5,6]. Extremely low-frequency electromagnetic fields can also modify the biophysical properties of cell membranes as shown by changes in the membrane permeability of carbonic anhydrase [7] and stimulation of the activity of Ca2+-activated potassium channels via increases in Ca2+ concentration and voltage-gated calcium channels [3,8,9]. We recently reported that ELF-EMF exposure significantly activated the voltage-gated sodium (Nav) channels of cerebellar GCs [10]. This activation is mediated by an increase in the intracellular concentration of arachidonic acid and involves EP receptor–mediated activation of the cAMP/PKA signalling pathway [10].
Melatonin (MT), which is synthesized and primarily secreted by the pineal gland, participates in many important physiological functions, including the control of seasonal reproduction, and influences the immune system and circadian rhythms [11,12]. In vitro and in vivo studies have revealed that MT and its metabolites can reduce oxidative stress-induced damage to proteins, lipids and nucleic acids in the presence of free radicals because of its free radical-scavenging properties [13–15]. Because it has been postulated that EMF exposure can affect the function of biological systems by inducing oxidative damage, the effects of MT on EMF-induced oxidative damage, cancer risk and neurodegeneration have been investigated [16,17]. Besides its direct free radical-scavenging properties, MT has been shown to modulate apoptosis caused by wireless (2.45 GHz)-induced oxidative stress through cation channels, such as transient receptor potential (TRP) and voltage-gated Ca2+ channels in neurons and transfected cells [18,19]. In addition, MT modulates the delay in outward rectifying K+ channels resulting in the promotion of cerebellar GC migration or the protection of cerebellar GCs against apoptosis [20,21]. However, there have been relatively few studies concerning the effect of MT on Na+ channels, especially EMF-induced Na+ channels activity.
Voltage-gated sodium channels are one of the primary classes of ion channels responsible for driving neuronal excitability in both the central and the peripheral nervous system. Voltage-gated sodium channels are clinically important because they play an important role in the generation of neuronal activity, and alterations in Nav channels are key factors in a number of pathologies [22]. Previous studies from other groups have revealed that Nav channels participate in the rising phase of the neuronal action potential and contribute to many cellular functions, including apoptosis, motility and secretory membrane activity [22,23]. Moreover, EMF exposure was recently reported to modulate neuronal excitation and neurogenesis, which may be related to Nav channel activity [24,25]. Our previous data have demonstrated that ELF-EMF exposure significantly activates the Nav channels of cerebellar GCs, which might be an important effect of EMF on neuronal excitation in the cerebellar GCs. Thus, a thorough investigation of the influence of ELF-EMF on Nav channels and the corresponding mechanism of action could elucidate the ELF-EMF-induced biological effects on brain physiology, pathogenesis and neural development. Therefore, it is interesting to address whether MT can modulate ELF-EMF-induced Nav channel activity.
This study was conducted to determine whether MT influences the Na+ channels of cerebellar GCs exposed to ELF-EMF and, if so, whether this effect is mediated by inactivation of the cAMP/PKA signalling pathway. The data presented in this report demonstrate that the activity of neuronal Na+ channels by ELF-EMF stimulation is significantly reversed by MT. Notably, the effect of MT on ELF-EMF-induced Na+ is not mediated by inhibition of the cAMP/PKA signalling pathway but by increasing intracellular calcium levels.
Materials and methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of Fudan University (Permit Number: 20090614-001). All surgery was performed under sodium pentobarbital anaesthesia, and all efforts were made to minimize suffering.
Primary cell culture
Cells were derived from the cerebellum of 7-day-old Sprague–Dawley rat pups as described previously [26]. Isolated cells were then plated onto 35-mm-diameter Petri dishes coated with poly-l-lysine (1 μg/ml) at a density of 2.5 × 105/cm2. Cultured cells were incubated at 37°C with 5% CO2 in DMEM supplemented with 10% foetal calf serum, glutamine (5 mM), insulin (5 μg/ml), KCl (25 mM) and 1% antibiotic–antimycotic solution. All experiments were carried out with cerebellar GCs grown for 6–8 days in culture (DIC). For Ca2+ imaging experiments, the cells were plated onto poly-l-lysine-coated glass coverslips (12 mm in diameter).
Electromagnetic field production
The system used to expose cerebellar GCs cells to electromagnetic fields was the same as that used in previous studies, with some revisions (I-ONE, Shanghai, China) [27,28]. Briefly, a 50-Hz magnetic field was generated by a pair of Helmholtz coils placed in opposition to each other. The coils were powered by a generator system that produced sinusoidal input voltage, and the magnetic flux densities could be regulated within the range of 0–1.0 mT. The device was powered by an AC power generator, and the EMF frequency and density were monitored by an EMF sensor that was connected to a digital multimeter. The geometry of the system assured a uniform field for the exposed cultured cells. The surfaces of the culture plates were parallel to the force lines of the alternating magnetic field in the solenoid. The air and culture medium temperatures were continuously monitored for the duration of experiments. The maximum temperature increase recorded in the cultures that were exposed to ELF-EMF (compared with non-exposed cultures) was 0.4 ± 0.1°C. To identify any possible influence of this increase on our results, we compared data obtained from cerebellar GCs cultured in two different CO2 incubators at temperature settings of 37.0 and 37.4°C, and the results were consistent. The incubator was keep closed throughout the EMF or non-EMF experiments to ensure that the conditions were stable. Non-EMF groups were incubated in the same incubator under the same conditions as those used for the exposed groups but without EMF.
Patch-clamp recordings
Whole-cell currents of granule neurons were recorded using a conventional patch-clamp technique. In 6–8 DIC cerebellar GCs, transient INa are largely unclamped because of an event generated at a site electrotonically distant from the soma and prone to escape from clamp control, presumably the axon [29]. Therefore, we chose those cells that were relatively isolated and only recorded currents without unclamped spike. Prior to current recordings, the culture medium was replaced with a bath solution containing (in mM) NaCl 145, KCl 2.5, HEPES 10, MgCl2 1 and glucose 10 (pH adjusted to 7.4 using NaOH). Soft glass recording pipettes were filled with an internal solution containing (in mM) CsCl 145, HEPES 10, MgCl2 2 and EGTA 5 (pH adjusted to 7.3 using CsOH). The pipette resistance was 5–6 MΩ after being filled with the internal solution. Whole-cell series resistances of 6–8 MΩ were routinely compensated by more than 70%. All currents were recorded using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) that was operated in voltage-clamp mode. A Pentium computer was connected to the recording equipment with a Digidata 1300 analogue-to-digital (A/D) interface. The current was digitally sampled at 100 μs (10 kHz), and the current signals were filtered using a 5-kHz, five-pole Bessel filter. The currents were corrected online for leak and residual capacitance transients using a P/4 protocol. Data acquisition and analysis were performed with pClamp10 software (Axon Instruments) and/or Origin8.1 (MicroCal, Northampton, MA, USA). All recordings were performed at room temperature (23–25C°).
Phosphorylated protein kinase A assay
The cells were lysed in HEPES-NP40 lysis buffer (20 mM HEPES, 150 mM NaCl, 0.5% NP-40, 10% glycerol, 2 mM EDTA, 100 μM Na3VO4, 50 mM NaF and 1% proteinase inhibitor cocktail at pH 7.5) on ice for 30 min. After centrifugation, the supernatant was mixed with 2× sodium dodecyl sulphate loading buffer and boiled for 5 min. The proteins were separated on a 10% polyacrylamide gel, transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA), blocked with 10% non-fat milk and incubated at 4°C overnight with a rabbit polyclonal antibody against the phosphorylated form of the PKA catalytic subunits (1:1000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) or a rabbit monoclonal antibody against GAPDH (1:1000; KangCheng, China). After extensive washing with TBST, the membrane was incubated with horseradish peroxidase–conjugated antimouse or anti-rabbit IgG (1:10,000; KangChen Bio-Tech, Shanghai, China) for 2 hrs at room temperature. Chemiluminescent signals were generated using a SuperSignal West Pico trial kit (Pierce, Rockford, IL, USA) and detected using a ChemiDoc XRS System (Bio-Rad Laboratories Inc., Hercules, CA, USA). The protein measurements were normalized with GAPDH and control/GAPDH as 1.0.
Measurement of intracellular Ca2+ levels
Intracellular Ca2+ levels were detected by single cell fura-2 AM fluorescence intensity as described by Grynkiewicz [30]. Briefly, cultured cerebellar GCs were rinsed twice with balanced salt solution (BSS), then incubated at 37°C for 45 min. in the presence of 5 μM fura-2 AM (0.1% dimethylsulfoxide (DMSO) in BSS), washed twice again with BSS and incubated for an additional 20 min. prior to imaging. The BSS was composed of (in mM) NaCl 145, KCl 2.5, HEPES 10, MgCl2 1, glucose 10, CaCl2 2. The coverslips were transferred to a coverslip chamber, which was mounted on the stage of an inverted phase contrast microscope (Nikon, Eclipse Ti, Japan). Fresh BSS was added to the chamber, and the data were collected at 4-sec. intervals throughout the experiment. The excitation wavelengths for fura-2 AM were 340 and 380 nm, with emission at 505 nm. Baseline [Ca2+]i was determined for 60 sec. immediately prior to the addition of high K+ solution (27 mM KCl). Quantification of the fluorescence intensity was performed with Metafluor software (Universal Imaging Corporation, Downingtown, PA, USA).
Statistical analysis
Statistical analysis was performed with Student's t-test with non-paired or paired comparisons, as relevant. The values are given as the means ± SEM, with n representing the number of cells tested. A value of P < 0.05 was considered a significant difference between groups. When multiple comparisons were made, the data were analysed by one-way anova followed by the Tukey and Fisher LSD test for samples of more than two using Originpro software (OriginLab Corporation, Northampton, MA, USA).
Results
First, we investigated the effect of MT on the influence of ELF-EMF on the INa of cerebellar GCs. An INa was elicited by a depolarization step to −20 mV from the holding potential of −100 mV. Our previous study demonstrated that the increase in INa amplitude induced by ELF-EMF exposure was time dependent, and when cerebellar GCs were exposed to 1 mT ELF-EMF for 60 min., the amplitude of the INa increased significantly and was stable [10]. Moreover, exposure cell or neuron to 1–5 mT EMF with short time was reported by Bai and Moghadam's studies [31,32]. Therefore, we chose the same parameters of 1 mT ELF-EMF for 60 min. in this study. Similar to our previous report [10], when cerebellar GCs were exposed to 1 mT ELF-EMF for 60 min., the amplitude of the INa increased by ∽62.5 ± 6.6% (n = 25, P < 0.05) compared with cells that were not exposed to ELF-EMF (n = 33, Fig.1A). Melatonin significantly inhibited the increase in INa induced by ELF-EMF. In the presence of 1 μM or 5 μM MT, 60 min. of ELF-EMF exposure only increased the INa by 22.0 ± 5.9% and 8.9 ± 4.4% (n = 15 and 16, P < 0.05), respectively, which is significantly different from 60 min. of ELF-EMF exposure alone. However, MT alone did not modify INa activity. There was no significant difference from the control group when 5 μM MT was added to the bath solution (Fig.1B).
Figure 1.

The effects of melatonin (MT) on increased INa densities induced by exposure to extremely low-frequency electromagnetic field (ELF-EMF). (A) Superimposed INa evoked by a 20-msec. depolarizing pulse from a holding potential 100 to −20 mV. Current traces were obtained from cerebellar granule cells exposed to 1 mT ELF-EMF for 60 min. in the presence or absence of MT (1 and 5 μM). (B) Statistical analysis of the effects of MT on increased INa densities induced by exposure to ELF-EMF. The data are reported as the means ± SEM from 16 to 25 cells. *P < 0.05 compared with control (non-ELF-EMF) using Student's t-test. #P < 0.05 compared with the corresponding ELF-EMF control (without MT) using Student's t-test.
The inhibitory effect of MT on the ELF-EMF-induced INa activity could be mimicked by the MT2 receptor agonist, IIK-7 (Fig.2A). Similar to MT, IIK7 alone did not affect INa activity. In the presence of 10 μM IIK7, the increase in INa induced by ELF-EMF exposure was reduced from 46.8 ± 3.5% (n = 20) to −9.2 ± 6.5% (n = 27). This indicates that when IIK7 was used, the INa densities obtained from exposed cerebellar GCs were reduced by 9.2 ± 6.5% compared with that of non-ELF-EMF exposure group. Blocking MT2 with 4-P-PDOT eliminated the inhibitory effect of MT on ELF-EMF-induced INa activity. Pre-incubation of cerebellar GCs with 4-P-PDOT (10 μM) in the medium resulted in an increase in the INa amplitude after exposure to ELF-EMF by 56.3 ± 3.0% (n = 24). These data were significantly different from that obtained from cells exposed to ELF-EMF with MT (n = 25, Fig.2A). However, 4-P-PDOT (10 μM) itself did not modify the INa amplitude (Fig.2B).
Figure 2.
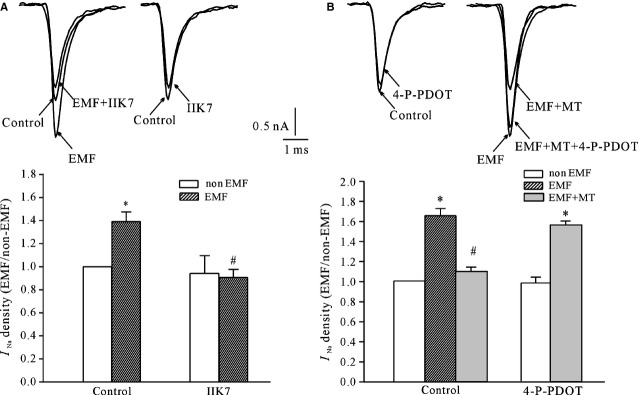
The effects of MT2 receptor agonist and antagonist on the melatonin (MT)-mediated inhibitory effects on extremely low-frequency electromagnetic field (ELF-EMF) exposure–induced INa enhancement. (A) Current traces and statistical analysis show the effects of the selective MT2R agonist IIK7 on INa obtained from ELF-EMF- and non-ELF-EMF-exposed groups. (B) Current traces and statistical analysis show the effects of the selective MT2R antagonist 4-P-PDOT on MT-induced inhibition of INa in ELF-EMF-exposed cerebellar granule cells. *P < 0.05 compared with the corresponding control (non-ELF-EMF exposed) using Student's t-test. #P < 0.05 compared with ELF-EMF exposed without MT using Student's t-test.
Our previous data indicated that ELF-EMF exposure significantly shifted the voltage dependence of the steady-state activation curve of INa, but the steady-state inactivation curve of INa did not significantly shift upon exposure to ELF-EMF [10]. We further investigated whether the inhibitory effects of MT on ELF-EMF-induced INa activity were because of modulation of the voltage-gating properties of INa channels. The activation properties of INa in cerebellar GCs following exposure to ELF-EMF were studied using the appropriate voltage protocols. INa was evoked by a 20-msec. depolarizing pulse from a holding potential of −100 mV to potentials between −70 and 20 mV, with 5-mV steps in 5-sec. intervals (Fig.3A). A value for the steady-state activation of INa was then obtained by normalizing the conductance as a function of the command potential; conductance was calculated as GNa = INa/(Vm1/2 − Vrev). The data points were fitted to the Boltzmann function GNa/GNa-max = 1/{1+exp [(Vm1/2 − Vm)/k]}, and the half-activation potentials were calculated. Figure3B illustrates the steady-state activation curve of INa obtained from cerebellar GCs that were exposed to ELF-EMF with or without MT. The half-activation potentials obtained from the control GC group was −43.3 ± 2.1 mV, which shifted to −48.8 ± 1.3 mV (n = 14, P < 0.05) when cerebellar GCs were exposed to ELF-EMF. In the presence of MT, the half-activation potentials were −44.2 ± 1.1 mV for GCs with no ELF-EMF exposure (n = 6) and −47.7 ± 1.7 mV for GCs exposed to ELF-EMF (n = 12). These data suggest that MT did not modify the steady-state activation property of the INa channels of the cerebellar GCs regardless of whether they were exposed to ELF-EMF.
Figure 3.
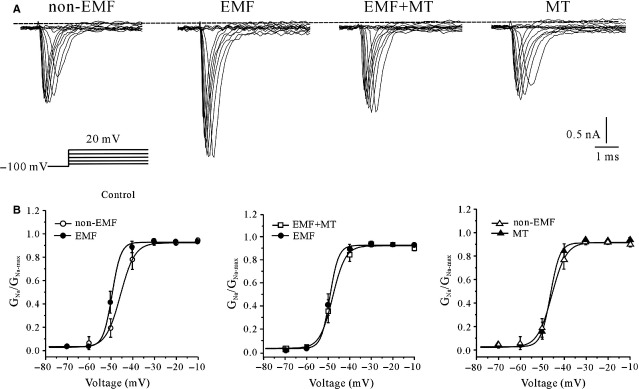
The effect of melatonin (MT) on the steady-state activation property of INa channels in extremely low-frequency electromagnetic field (ELF-EMF)-exposed and non-ELF-EMF-exposed cerebellar granule cells (GCs). (A) Representative superimposed INa evoked by steady-state activation voltage protocol obtained from non-ELF-EMF- and ELF-EMF-exposed cerebellar GCs in the presence and absence of MT. The cells were held at −100 mV and depolarized in 5-mV steps from −70 to 20 mV with intervals of 5 sec. (B) The voltage-dependent activation curve of INa obtained from non-ELF-EMF- and ELF-EMF-exposed cerebellar GCs in the presence and absence of MT. The data are expressed as the means ± SEM from 13 to 12 cells.
Our previous study showed that the INa of cerebellar GCs was enhanced by activation of PKA [33], and a significant increase in the intracellular levels of phosphorylated PKA (pPKA), as measured using an immunoblot assay, was observed following ELF-EMF exposure [10]. We thus studied the effect of MT on intracellular pPKA levels to address whether MT functioned by inhibiting the PKA activity. Unexpectedly, both MT and IIK-7 increased the intracellular pPKA by 15.1 ± 5.0% and 16.2 ± 7.3% respectively (n = 8, P < 0.05; Fig.4A and B). The presence of MT or IIK-7 did not inhibit the ELF-EMF exposure-induced increase in intracellular pPKA (Fig.4A and B), which was 26.3 ± 8.7% for MT and 29.4 ± 7.3% for IIK-7 (n = 8, P < 0.05). These results suggest that PKA activation is not associated with the inhibitory effect of MT on the ELF-EMF exposure–induced increase in INa channel activity.
Figure 4.
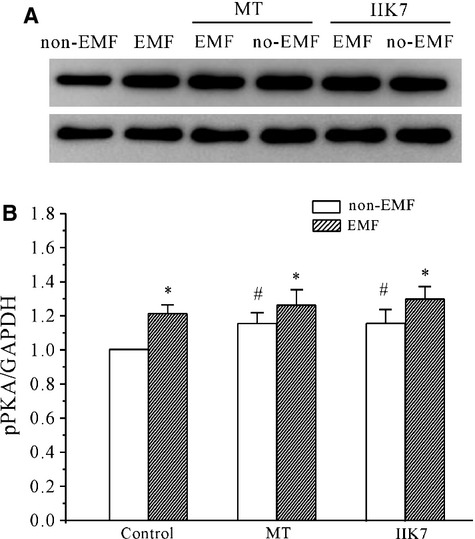
The effect of melatonin (MT) and IIK-7 on the PKA activity in extremely low-frequency electromagnetic field (ELF-EMF)- and non-ELF-EMF-exposed cerebellar granule cells (GCs) measured by Western blot analysis. (A) Representative samples show the effects of MT and IIK-7 on PKA levels. (B) The statistical analysis of the effects of MT and IIK-7 on PKA levels in ELF-EMF- and non-ELF-EMF-exposed cerebellar GCs. *P < 0.05 compared with non-ELF-EMF-exposed controls using Student's t-test. #P < 0.05 compared with the corresponding non-ELF-EMF-exposed control without MT or IIK-7 using Student's t-test.
It has previously been reported that Ca2+/CaM can modulate voltage-gated Na+ channels in neurons and muscles [34,35]. We therefore examined the effects of KN-93, a Ca2+/CaMKII blocker, on the inhibitory effect of MT on the ELF-EMF exposure–induced increase in INa channel activity. KN-93 alone did not modify the INa amplitude. In the presence of KN-93 (10 μM), the INa amplitude after ELF-EMF exposure with MT was increased from 8.9 ± 4.4% to 49.4 ± 17.1% (n = 19; Fig.5A), which was significantly different from the results obtained without KN-93 (P < 0.05), suggesting that the Ca2+/CaM pathway is associated with the inhibitory effect of MT on the ELF-EMF exposure–induced increase in INa. We then tested the effect on ELF-EMF exposure–induced increase in INa by treatment of C6-ceramide, which increases Ca2+ release through the ryanodine-sensitive Ca2+ receptor [35]. Based on our results, C6-ceramide could mimic the effect of MT and decreased the ELF-EMF-induced inhibitory effect in INa from 71.2 ± 8.0% to 16.8 ± 11.7% (n = 11, Fig.5B). In rat cerebellar GCs, intracellular Ca2+ is mainly released by the ryanodine-sensitive Ca2+ receptor pathway [35]. Therefore, we used ruthenium red, a ryanodine-sensitive receptor blocker, to probe whether the inhibitory effect of MT on the ELF-EMF exposure–induced increase in INa is through ryanodine-sensitive Ca2+ receptor–induced calcium release. Consistent with previous reports by Claire O. Malecot et al. [36], incubation of rat cerebellar GCs with ruthenium red (10 μM) alone significantly reduced INa amplitude. On the other hand, blocking ryanodine-sensitive Ca2+ receptor by ruthenium red significantly eliminated the inhibitory effect of MT on the ELF-EMF exposure–induced increase in INa: in the presence of ruthenium red (10 μM), the INa amplitude after ELF-EMF exposure with MT was still increased 85.4 ± 8.1% (n = 9) compared with the group treated with ruthenium red alone (Fig.5C).
Figure 5.
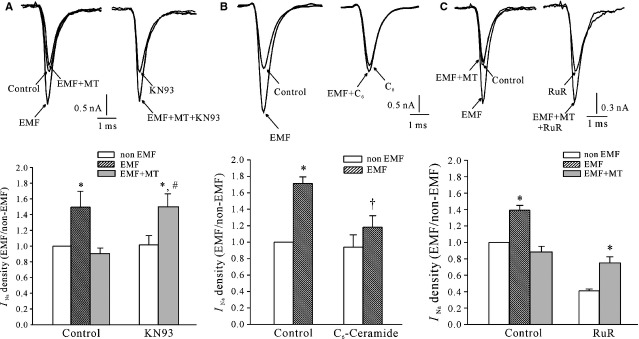
Current traces and statistical analysis show the effects of the Ca2+/CaM pathway on melatonin (MT)-induced inhibition of INa in extremely low-frequency electromagnetic field (ELF-EMF)-exposed cerebellar granule cells. (A) Representative superimposed INa traces and statistical analysis showing that KN-93 significantly abolished the effect of MT on the EMF-induced INa increase. (B) Representative superimposed INa traces and statistical analysis showed that C6-ceremade (C6) could mimic the effect of MT on the EMF-induced INa increase. (C) Representative superimposed INa traces and statistical analysis showed that ruthenium red (Rud) significantly abolished the effect of MT on the EMF-induced INa increase. *P < 0.05 compared with the non-ELF-EMF-exposed controls using Student's t-test. #P < 0.05 compared with the corresponding control (ELF-EMF exposed to MT) using Student's t-test. †P < 0.05 compared with the corresponding control without C6-ceramide treatment using Student's t-test.
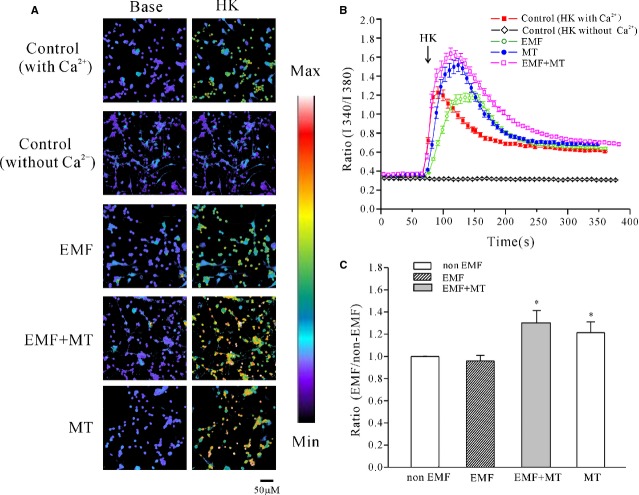
To confirm that intracellular Ca2+ was associated with the inhibitory effect of MT on ELF-EMF-induced INa activity, we used direct Ca2+ imaging using the calcium-sensitive fluorescent dye fura-2 AM to examine the potential effects of MT on intracellular calcium levels in cerebellar GCs. Because MT did not affect the basal level of intracellular calcium of cerebellar GCs, we used a high K+-solution (27 mM KCl) to depolarize neurons and activate voltage-dependent Ca2+ channels (VGCCs), leading to a rapid increase in the calcium concentration [37]. In control neurons, depolarization stimulation by high K+ evoked an acute elevation of intracellular Ca2+ levels with an increase in the F340/F380 ratio from 0.37 ± 0.006 to 1.25 ± 0.061 (n = 26), depicting as a shift from purple to red. After exposure to ELF-EMF, the high K+ stimulation evoked a similar intracellular Ca2+ level increase from 0.35 ± 0.016 to 1.21 ± 0.055 (n = 25), which was not significantly different compared with the group with non-ELF-EMF exposure (Fig.6A and B). Melatonin did not increase the basal intracellular Ca2+ level, but it significantly elevated the intracellular Ca2+ level evoked by high K+ stimulation in cerebellar GCs that were either exposed to ELF-EMF or not (Fig.6A and B). In the presence of MT, the intracellular Ca2+ F340/F380 ratio evoked by high K+ stimulation was significantly increased in cerebellar GCs that were either exposed to ELF-EMF or not to 1.62 ± 0.064 (n = 35) and 1.52 ± 0.055 (n = 31), respectively (Fig.6B), which was significantly different from the 1.21 ± 0.055 and 1.25 ± 0.061 recorded in the absence of MT. Increases in intracellular Ca2+ levels were calculated as a percentage of the control based on the F340/F380 ratio and were 29.6 ± 11.0% and 21.2 ± 9.6%, respectively, (Fig.6C) for cerebellar GCs that were either exposed to ELF-EMF or not. However, when Ca2+ was removed from bath solution, depolarization stimulation by high K+ did not increase the intracellular Ca2+ levels, suggesting that extracellular Ca2+ influx was needed for the depolarization-induced intracellular Ca2+ increase (Fig.6A–C).
Figure 6.
Effect of melatonin (MT) on the increase in intracellular Ca2+ level induced by high K+ in control cells and cells exposed to extremely low-frequency electromagnetic field (ELF-EMF). (A) [Ca2+] imaging obtained before and after depolarizing membranes by acute perfusion of a solution containing 27 mM K+ from ELF-EMF exposure and control cerebellar granule cells (GCs) in the presence or absence of MT. Changes in the fura-2 AM fluorescence excitation ratios with increasing [Ca2+] are depicted as a switch from purple to red; scale bar, 50 μm. (B) Changes in intracellular Ca2+ concentrations upon application of a depolarizing stimulus as measured by quantification of fluorescence excitation ratios. Each arrow represents a 30-sec. perfusion with a depolarizing solution containing 27 mM K+. (C) Statistical analysis of intracellular Ca2+ level obtained fromELF-EMF-exposed and control cerebellar GCs in the presence or absence of MT. The data were obtained from four independent experiments and are the means ± SEM; *P < 0.05 compared with the corresponding control by unpaired t-test.
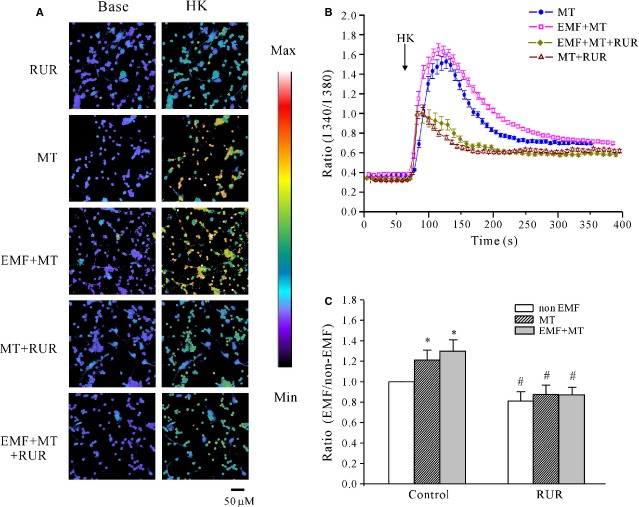
We also used ruthenium red to address whether the MT-induced increase in calcium release occurred through the ryanodine-sensitive Ca2+ receptor. Pre-incubation of cerebellar GCs with 20 μM ruthenium red alone induced a slight reduction in the F340/F380 ratio stimulated by high K+ from 1.25 ± 0.061 to 0.91 ± 0.050 (n = 28; Fig.7A and B), which was an inhibition of 19.2 ± 8.8% compared with the control group (Fig.7C); in the presence of ruthenium red, the increase in the F340/F380 ratio induced by MT was reduced from 1.52 ± 0.055 to 1.01 ± 0.037 (n = 24) in the control group. Similarly, ruthenium red also significantly inhibited the effect of MT on intracellular Ca2+ release after ELF-EMF exposure, which resulted in a decrease in the F340/F380 ratio from 1.62 ± 0.064 to 1.07 ± 0.078 (n = 34; Fig.7C).
Figure 7.
Effects of blocking ryanodine-sensitive receptors on melatonin (MT)-induced increase in intracellular Ca2+ level in control cells and cells exposed to extremely low-frequency electromagnetic field (ELF-EMF). (A) [Ca2+] imaging obtained before and after depolarizing membranes by acute perfusion of a solution containing 27 mM K+ from ELF-EMF-exposed cells with MT in the presence or absence of Ruthenium Red (RuR, 20 μM); scale bar, 50 μm. (B) Changes in intracellular calcium concentrations upon application of RuR. Each arrow represents a 30-sec. perfusion with a depolarizing solution containing 27 mM K+. (C) Statistical analysis of intracellular Ca2+ levels obtained after ELF-EMF exposure with MT in the presence or absence of RuR. The data are the means ± SEM obtained from four independent experiments; *P < 0.05 compared with non-ELF-EMF-exposed cells without MT using Student's t-test. #P < 0.05 compared with the corresponding control by unpaired t-test.
Discussion
Although several ion channels, such as delayed rectifier outward K+ current (IK), TRPM-2 and voltage-dependent L- type Ca2+ channels, are known to be modulated by MT [18–21], the effects of MT on Nav channels are poorly understood. Although MT has previously been reported to protect cells against EMF stimulus, whether it modulates the activity of ion channels induced by EMF exposure is poorly understood. Here, we report for the first time that MT itself was not able to modify INa, but might inhibit INa enhancement resulting from ELF-EMF exposure in cerebellar GCs by increasing the concentration of intracellular Ca2+.
It is well known that the effects of exposure to EMF differ significantly based on the ELF-EMF exposure intensities and the exposure time. Our previous study indicates that exposure of cerebellar GCs to ELF-EMF (1 mT) for short time (10–60 min.) significantly increases the amplitude of the INa. Moreover, exposure to ELF-EMF induces similar effects on INa in rat cerebellar GCs regardless the condition, whether it is 1 mT stimulation for a short time or 0.4 mT stimulation for a longer time. Notably, it is generally believed that short-term changes induced by EMF are mediated by modifications in enzyme activity in the cytosol or the membrane [4,38,39], while the long-term exposure to EMF may induce changes in nuclear functions such as gene transcription and cell cycle regulation [27,40]. To avoid the influence of multiple factors because of long-term EMF exposure, we performed all our experiments at 1 mT EMF exposure for a short time, which we believe that it is suitable to assess the effect of ELF-EMF on intracellular signalling pathways.
Although some of the main functions attributed to MT include its role as a free radical scavenger and its indirect antioxidant properties [41], studies have shown that MT can interact with specific receptors to exert its biological effect [42]. Our previous study demonstrated that activation of the MT2 receptor (MT2R) by MT and a low concentration of 2-iodomelatonin increased the delayed-rectified outward K+ current (IK) [43] and improved cerebellar GC migration [21]. In contrast, a high concentration of 2-iodomelatonin could inhibit the IK recorded from cerebellar GCs by activating the MT1 receptor, which protects the neurons against apoptotic stimulus [20]. In this study, a selective MT2 agonist could mimic the effect of MT on EMF-induced INa enhancement, which could be blocked by a selective MT2 antagonist, suggesting that it is highly likely that the inhibitory effect of MT on EMF-induced INa enhancement was mediated by MT2R, and did not directly result from the antioxidant properties of MT. This is consistent with our previous findings on the effect of MT on the potassium current in cerebellar GCs [20,21,43].
Among the possible mechanisms underlying the inhibitory effect of MT on EMF-induced INa increase, we first considered the involvement of the MT2-mediated cAMP/PKA pathway because EMF-induced INa increase is thought to occur via the cAMP/PKA pathway [10], and negative modulation of cAMP/PKA activation by MT has previously been reported [21]. However, the experimental data presented here show that MT did not inhibit PKA activity; instead, it induced a slight increase in PKA activity. Furthermore, MT was not able to inhibit the PKA activity induced by EMF exposure. In addition, MT did not change the steady-state activity of INa channels in the control cerebellar GCs or in EMF-exposed cerebellar GCs, indicating that a non-phosphorylation-dependent mechanism is involved. Together, these data suggest that the ability of MT to counteract the effect of EMF on INa activity does not occur through the inhibition of EMF-induced PKA activity. MT failed to negatively modulate PKA activity in this study, which is different from what has previously been reported [21]. This effect might be as a result of the higher concentration of MT used in this study. Transfection experiments have demonstrated that when expressed in the same cell type, MT1 and MT2 receptors may couple to different signalling pathways [44]. Both our work and that of Marta indicate that the presence of native MT1/MT2 receptors in mouse and rat cerebellar GCs mediates the effects of MT on intracellular signalling pathways [20,21,45]. Thus, it is possible that 1–5 μM MT may activate both MT1 and MT2 receptors simultaneously, thereby inducing a coordinated and integrated effect on PKA. Therefore, the effect of MT on the PKA pathway was no longer evident.
In addition to the cAMP/PKA pathway, Ca2+ has been proposed to regulate Na+ channels through the action of calmodulin (CaM) bound to an isoleucine–glutamine motif in the C terminus of the Na+ channel subunit [46]. Previous studies indicate that MT modulates the Ca2+/CaM signalling pathway either by changing the intracellular calcium concentration ([Ca2+]i) via activation of its G-protein coupled membrane receptors or through a direct interaction with CaM [47,48]. MT was shown to be able to reverse cytosolic Ca2+ evoked by H2O2 stimuli [19]. In rat cerebellar GCs, intracellular Ca2+ is mainly released by the ryanodine-sensitive Ca2+ receptor pathway, which might maintain INa at a lower level [34]. In this study, the Ca2+/CaMKII and ryanodine-sensitive Ca2+ receptor blocker significantly abolished the effect of MT on the EMF-induced INa increase, providing evidence for the involvement of the Ca2+/CaM pathway. Interestingly, MT did not increase basal Ca2+ release, but significantly increased high K+ evoked intracellular Ca2+ levels, which was thought to result from membrane depolarization [49]. Furthermore, the increase in intracellular Ca2+ evoked by MT was inhibited when the ryanodine-sensitive Ca2+ receptor was blocked with ruthenium red. It is thus highly likely that MT activated the MT2R-mediated Ca2+ channels and improved extracellular Ca2+ influx, which consequently stimulated the release of Ca2+ through the ryanodine-sensitive Ca2+ receptor, by which the EMF-induced INa increase was reversed.
However, our previous study with Western blotting has revealed [10] that ELF-EML increases the current density by increasing the NaV channel protein expression on cerebellar GCs membrane, although the content of expression is lower. Whether increase in Nav channel protein expression or else mechanism is involved in MT-induced and Ca2+/CaM-dependent modulation of INa amplitude remains unclear, and further study is necessary.
Taken together, our data suggest that MT eliminated the EMF-induced INa increase through Ca2+ influx–induced Ca2+ release but not by abolishing EMF-induced PKA activity. However, we noticed that MT itself did not affect the amplitude of INa in control cerebellar GCs, although it was able to increase the intracellular Ca2+ levels evoked by high K+ depolarization stimuli. This might be because basal intracellular Ca2+ released from ryanodine-sensitive receptors maintain the INa at a low enough level in rat cerebellar GCs [34] that MT-induced Ca2+ release is not able to reduce the INa densities further. However, when cerebellar GCs were exposed to EMF with MT, high K+-mediated Ca2+ release was significantly enhanced, and MT reversed the EMF-induced increase in INa amplitude. This observation was consistent with previous studies that indicated that most of the effects of MT on second messengers or effectors require prior stimulatory input [50,51]. It is highly likely that with EMF exposure as stimulatory input, the effect of MT on INa could be fully realized.
Currently, there is widespread use of EMR-emitting devices in industrial, scientific, medical and domestic applications, with the potential for leakage of such radiation into the environment [52]. The effects of ELF-EMF on nerve cells have been extensively studied in various organisms [8,53]. Although the reported results are variable or contradictory because of differences in the experimental conditions and in the density and/or duration of EMF exposure, EMF has recently been reported to modulate neuronal excitatory functions and neurogenesis [8,24,25]. The findings from our molecular-level analysis of the protective effects of MT on INa produced by exposure to ELF-EMF might provide evidence for an important effect of MT on neuronal excitation during EMF exposure in cerebellar GCs. Nonetheless, the modulatory effects on neuronal excitatory functions caused by exposure to ELF-EMF are complicated and varied. In addition, the functional mechanism of MT and its receptors in native cerebellar GCs are complex. Therefore, further exploration is required to comprehensively analyse the biological protective effects of MT and its mechanism on neurons that are exposed to ELF-EMF.
Acknowledgments
The study was supported by a grant from the National Basic Research Program of China (2011CB503703) and the Shanghai Leading Academic Discipline Project (B111). Qian-Ru zhao was supported by the National Talent Training Fund in Basic Research of China (No. J1210012).
Conflicts of interest
The authors confirm that there are no conflicts of interest.
Author contribution
Dong-Dong Liu performed experiments, analysed data, interpreted results of experiments and prepared figures; Zheng Ren helped to perform experiments and analyse data; Guang Yang and Qian-Ru zhao helped to perform experiments, analyse data and interpret results of experiments; Yan-Ai Mei contributed to the design of research, drafted the manuscript and approved the final version of manuscript.
References
- 1.Cui Y, Ge Z, Rizak JD, et al. Deficits in water maze performance and oxidative stress in the hippocampus and striatum induced by extremely low frequency magnetic field exposure. PLoS ONE. 2012;7:e32196. doi: 10.1371/journal.pone.0032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luukkonen J, Liimatainen A, Hoyto A, et al. Pre-exposure to 50 Hz magnetic fields modifies menadione-induced genotoxic effects in human SH-SY5Y neuroblastoma cells. PLoS ONE. 2011;6:e18021. doi: 10.1371/journal.pone.0018021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piacentini R, Ripoli C, Mezzogori D, et al. Extremely low-frequency electromagnetic fields promote in vitro neurogenesis via upregulation of Ca(v)1-channel activity. J Cell Physiol. 2008;215:129–39. doi: 10.1002/jcp.21293. [DOI] [PubMed] [Google Scholar]
- 4.Arendash GW, Mori T, Dorsey M, et al. Electromagnetic treatment to old Alzheimer's mice reverses beta-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS ONE. 2012;7:e35751. doi: 10.1371/journal.pone.0035751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dibirdik I, Kristupaitis D, Kurosaki T, et al. Stimulation of Src family protein-tyrosine kinases as a proximal and mandatory step for SYK kinase-dependent phospholipase Cgamma2 activation in lymphoma B cells exposed to low energy electromagnetic fields. J Biol Chem. 1998;273:4035–9. doi: 10.1074/jbc.273.7.4035. [DOI] [PubMed] [Google Scholar]
- 6.Sun WJ, Chiang H, Fu YT, et al. Exposure to 50 Hz electromagnetic fields induces the phosphorylation and activity of stress-activated protein kinase in cultured cells. Electro Magnetobiol. 2001;20:415–23. [Google Scholar]
- 7.Ramundo-Orlando A, Mattia F, Palombo A, et al. Effect of low frequency, low amplitude magnetic fields on the permeability of cationic liposomes entrapping carbonic anhydrase: II. No evidence for surface enzyme involvement. Bioelectromagnetics. 2000;21:499–507. doi: 10.1002/1521-186x(200010)21:7<499::aid-bem3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Marchionni I, Paffi A, Pellegrino M, et al. Comparison between low-level 50 Hz and 900 MHz electromagnetic stimulation on single channel ionic currents and on firing frequency in dorsal root ganglion isolated neurons. Biochim Biophys Acta. 2006;1758:597–605. doi: 10.1016/j.bbamem.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17:958–65. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He YL, Liu DD, Fang YJ, et al. Exposure to extremely low-frequency electromagnetic fields modulates Na+ currents in rat cerebellar granule cells through increase of AA/PGE2 and EP receptor-mediated cAMP/PKA pathway. PLoS ONE. 2013;8:e54376. doi: 10.1371/journal.pone.0054376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardeland R, Madrid JA, Tan DX, et al. Melatonin, the circadian multioscillator system and health: the need for detailed analyses of peripheral melatonin signaling. J Pineal Res. 2012;52:139–66. doi: 10.1111/j.1600-079X.2011.00934.x. [DOI] [PubMed] [Google Scholar]
- 12.Calvo JR, Gonzalez-Yanes C, Maldonado MD. The role of melatonin in the cells of the innate immunity: a review. J Pineal Res. 2013;55:103–20. doi: 10.1111/jpi.12075. [DOI] [PubMed] [Google Scholar]
- 13.Reiter RJ, Tan DX, Manchester LC, et al. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34:237–56. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- 14.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 15.Galano A, Tan DX, Reiter RJ. On the free radical scavenging activities of melatonin's metabolites, AFMK and AMK. J Pineal Res. 2013;54:245–57. doi: 10.1111/jpi.12010. [DOI] [PubMed] [Google Scholar]
- 16.Naziroglu M, Tokat S, Demirci S. Role of melatonin on electromagnetic radiation-induced oxidative stress and Ca2+ signaling molecular pathways in breast cancer. J Recept Signal Transduct Res. 2012;32:290–7. doi: 10.3109/10799893.2012.737002. [DOI] [PubMed] [Google Scholar]
- 17.Vijayalaxmi, Reiter RJ, Meltz ML, et al. Melatonin: possible mechanisms involved in its ‘radioprotective’ effect. Mutat Res. 1998;404:187–9. doi: 10.1016/s0027-5107(98)00112-2. [DOI] [PubMed] [Google Scholar]
- 18.Naziroglu M, Celik O, Ozgul C, et al. Melatonin modulates wireless (2.45 GHz)-induced oxidative injury through TRPM2 and voltage gated Ca(2+) channels in brain and dorsal root ganglion in rat. Physiol Behav. 2012;105:683–92. doi: 10.1016/j.physbeh.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Celik O, Naziroglu M. Melatonin modulates apoptosis and TRPM2 channels in transfected cells activated by oxidative stress. Physiol Behav. 2012;107:458–65. doi: 10.1016/j.physbeh.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Jiao S, Wu MM, Hu CL, et al. Melatonin receptor agonist 2-iodomelatonin prevents apoptosis of cerebellar granule neurons via K(+) current inhibition. J Pineal Res. 2004;36:109–16. doi: 10.1046/j.1600-079x.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu LY, Hoffman GE, Fei XW, et al. Delayed rectifier outward K+ current mediates the migration of rat cerebellar granule cells stimulated by melatonin. J Neurochem. 2007;102:333–44. doi: 10.1111/j.1471-4159.2007.04669.x. [DOI] [PubMed] [Google Scholar]
- 22.Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience. 2004;126:31–44. doi: 10.1016/S0306-4522(03)00425-1. [DOI] [PubMed] [Google Scholar]
- 23.Ding Y, Brackenbury WJ, Onganer PU, et al. Epidermal growth factor upregulates motility of Mat-LyLu rat prostate cancer cells partially via voltage-gated Na+ channel activity. J Cell Physiol. 2008;215:77–81. doi: 10.1002/jcp.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldinucci C, Carretta A, Maiorca SM, et al. Effects of 50 Hz electromagnetic fields on rat cortical synaptosomes. Toxicol Ind Health. 2009;25:249–52. doi: 10.1177/0748233709103031. [DOI] [PubMed] [Google Scholar]
- 25.Cuccurazzu B, Leone L, Podda MV, et al. Exposure to extremely low-frequency (50 Hz) electromagnetic fields enhances adult hippocampal neurogenesis in C57BL/6 mice. Exp Neurol. 2010;226:173–82. doi: 10.1016/j.expneurol.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez B, Leroux P, Lamacz M, et al. Somatostatin receptors are expressed by immature cerebellar granule cells: evidence for a direct inhibitory effect of somatostatin on neuroblast activity. Proc Natl Acad Sci USA. 1992;89:9627–31. doi: 10.1073/pnas.89.20.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ongaro A, Varani K, Masieri FF, et al. Electromagnetic fields (EMFs) and adenosine receptors modulate prostaglandin E(2) and cytokine release in human osteoarthritic synovial fibroblasts. J Cell Physiol. 2012;227:2461–9. doi: 10.1002/jcp.22981. [DOI] [PubMed] [Google Scholar]
- 28.Varani K, Vincenzi F, Targa M, et al. Effect of pulsed electromagnetic field exposure on adenosine receptors in rat brain. Bioelectromagnetics. 2012;33:279–87. doi: 10.1002/bem.20704. [DOI] [PubMed] [Google Scholar]
- 29.Diwakar S, Magistretti J, Goldfarb M, et al. Axonal Na+ channels ensure fast spike activation and back-propagation in cerebellar granule cells. J Neurophysiol. 2009;101:519–32. doi: 10.1152/jn.90382.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- 31.Bai WF, Xu WC, Feng Y, et al. Fifty-Hertz electromagnetic fields facilitate the induction of rat bone mesenchymal stromal cells to differentiate into functional neurons. Cytotherapy. 2013;15:961–70. doi: 10.1016/j.jcyt.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Moghadam MK, Firoozabadi M, Janahmadi M. Effects of weak environmental magnetic fields on the spontaneous bioelectrical activity of snail neurons. J Membr Biol. 2011;240:63–71. doi: 10.1007/s00232-011-9344-z. [DOI] [PubMed] [Google Scholar]
- 33.Fang YJ, Zhou MH, Gao XF, et al. Arachidonic acid modulates Na+ currents by non-metabolic and metabolic pathways in rat cerebellar granule cells. Biochem J. 2011;438:203–15. doi: 10.1042/BJ20110569. [DOI] [PubMed] [Google Scholar]
- 34.Liu Z, Xu JG, Zhang H, et al. C(6)-ceramide inhibited Na(+) currents by intracellular Ca(2+) release in rat myoblasts. J Cell Physiol. 2007;213:151–60. doi: 10.1002/jcp.21106. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Fei XW, Fang YJ, et al. PLC-dependent intracellular Ca2+ release was associated with C6-ceramide-induced inhibition of Na+ current in rat granule cells. J Neurochem. 2008;106:2463–75. doi: 10.1111/j.1471-4159.2008.05562.x. [DOI] [PubMed] [Google Scholar]
- 36.Malecot CO, Bito V, Argibay JA. Ruthenium red as an effective blocker of calcium and sodium currents in guinea-pig isolated ventricular heart cells. Br J Pharmacol. 1998;124:465–72. doi: 10.1038/sj.bjp.0701854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutierrez-Martin Y, Martin-Romero FJ, Henao F, et al. Alteration of cytosolic free calcium homeostasis by SIN-1: high sensitivity of L-type Ca2+ channels to extracellular oxidative/nitrosative stress in cerebellar granule cells. J Neurochem. 2005;92:973–89. doi: 10.1111/j.1471-4159.2004.02964.x. [DOI] [PubMed] [Google Scholar]
- 38.Morelli A, Ravera S, Panfoli I, et al. Effects of extremely low frequency electromagnetic fields on membrane-associated enzymes. Arch Biochem Biophys. 2005;441:191–8. doi: 10.1016/j.abb.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Ravera S, Bianco B, Cugnoli C, et al. Sinusoidal ELF magnetic fields affect acetylcholinesterase activity in cerebellum synaptosomal membranes. Bioelectromagnetics. 2010;31:270–6. doi: 10.1002/bem.20563. [DOI] [PubMed] [Google Scholar]
- 40.Richard D, Lange S, Viergutz T, et al. Influence of 50 Hz electromagnetic fields in combination with a tumour promoting phorbol ester on protein kinase C and cell cycle in human cells. Mol Cell Biochem. 2002;232:133–41. doi: 10.1023/a:1014802005672. [DOI] [PubMed] [Google Scholar]
- 41.Jou MJ, Peng TI, Yu PZ, et al. Melatonin protects against common deletion of mitochondrial DNA-augmented mitochondrial oxidative stress and apoptosis. J Pineal Res. 2007;43:389–403. doi: 10.1111/j.1600-079X.2007.00490.x. [DOI] [PubMed] [Google Scholar]
- 42.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 43.Huan C, Zhou M, Wu M, et al. Activation of melatonin receptor increases a delayed rectifier K+ current in rat cerebellar granule cells. Brain Res. 2001;917:182–90. doi: 10.1016/s0006-8993(01)02915-8. [DOI] [PubMed] [Google Scholar]
- 44.Petit L, Lacroix I, de Coppet P, et al. Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3′-5′-monophosphate pathway. Biochem Pharmacol. 1999;58:633–9. doi: 10.1016/s0006-2952(99)00134-3. [DOI] [PubMed] [Google Scholar]
- 45.Imbesi M, Uz T, Dzitoyeva S, et al. Melatonin signaling in mouse cerebellar granule cells with variable native MT1 and MT2 melatonin receptors. Brain Res. 2008;1227:19–25. doi: 10.1016/j.brainres.2008.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young KA, Caldwell JH. Modulation of skeletal and cardiac voltage-gated sodium channels by calmodulin. J Physiol. 2005;565:349–70. doi: 10.1113/jphysiol.2004.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai J, Inscho EW, Yuan L, et al. Modulation of intracellular calcium and calmodulin by melatonin in MCF-7 human breast cancer cells. J Pineal Res. 2002;32:112–9. doi: 10.1034/j.1600-079x.2002.1844.x. [DOI] [PubMed] [Google Scholar]
- 48.Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–53. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Dolezal V, Lisa V, Tucek S. Effect of tacrine on intracellular calcium in cholinergic SN56 neuronal cells. Brain Res. 1997;769:219–24. doi: 10.1016/s0006-8993(97)00711-7. [DOI] [PubMed] [Google Scholar]
- 50.Vanecek J. Cellular mechanisms of melatonin action. Physiol Rev. 1998;78:687–721. doi: 10.1152/physrev.1998.78.3.687. [DOI] [PubMed] [Google Scholar]
- 51.Hazlerigg DG, Morgan PJ, Lawson W, et al. Melatonin inhibits the activation of cyclic AMP-dependent protein kinase in cultured pars tuberalis cells from ovine pituitary. J Neuroendocrinol. 1991;3:597–603. doi: 10.1111/j.1365-2826.1991.tb00324.x. [DOI] [PubMed] [Google Scholar]
- 52.Consales C, Merla C, Marino C, et al. Electromagnetic fields, oxidative stress, and neurodegeneration. Int J Cell Biol. 2012;2012:683897. doi: 10.1155/2012/683897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lisi A, Ciotti MT, Ledda M, et al. Exposure to 50 Hz electromagnetic radiation promote early maturation and differentiation in newborn rat cerebellar granule neurons. J Cell Physiol. 2005;204:532–8. doi: 10.1002/jcp.20322. [DOI] [PubMed] [Google Scholar]