Abstract
Behçet syndrome (BS) is a multisystem vasculitis that is most active during young adulthood, causing serious disability and significant impairment in quality of life. Differences in the disease course, severity, and organ involvement between patients, depending on the age at presentation and sex, makes it impossible to determine a single management strategy. The diversity and variability in the outcome measures used in clinical trials in BS makes it difficult to compare the results or inform physicians about the best management strategy for individual patients. There is a large unmet need to determine or develop validated outcome measures for use in clinical trials in BS that are acceptable to researchers and regulatory agencies. We conducted a systematic review to describe the outcomes and outcome measures that have been used in clinical trials in BS. This review revealed the diversity and variability in the outcomes and outcome measures and the lack of standard definitions for most outcomes and rarity of validated outcome tools for disease assessment in BS. This systematic literature review will identify domains and candidate instruments for use in a Delphi exercise, the next step in the development of a core set of outcome measures that are properly validated and widely accepted by the collaboration of researchers from many different regions of the world and from different specialties, including rheumatology, ophthalmology, dermatology, gastroenterology, and neurology.
Keywords: BEHÇET SYNDROME, OUTCOMES, ASSESSMENT, SYSTEMATIC REVIEW
Behçet syndrome (BS) is a multisystem vasculitis that affects both men and women during young adulthood with significant effects on quality of life (QOL); it can result in serious disability and premature death. Manifestations include mucocutaneous lesions such as oral ulcers, genital ulcers, papulopustular and nodular lesions, joint involvement (usually in the form of a self-limited monoarthritis or arthralgia), eye involvement typically manifesting as panuveitis that may lead to blindness if left untreated, vascular involvement causing arterial aneurysms that may be lethal, deep venous thrombi, neurologic involvement that can cause permanent disability, and gastrointestinal (GI) involvement that is often indistinguishable from inflammatory bowel disease. Given its pathophysiology and spectrum of manifestations, BS is best classified as a form of vasculitis. There are many reasons to call it a syndrome rather than a disease, in particular the considerable variation in disease presentation depending on demography and geography1.
The disease course, severity, and types of organ involvement vary substantially among patients depending on their age and sex and the age of onset. Thus, it is impossible to determine a single management strategy2. Several clinical trials have been conducted in patients with BS, addressing different types of organ involvement such as mucocutaneous or ocular disease3. However, most of the outcome measures used in these trials were neither validated nor widely accepted, leading investigators to add to the clutter by creating their own definitions of activity, severity, or response. The diversity and variability in the outcome measures used in trials of BS make it difficult to compare trial results, combine findings into metaanalyses, or guide physicians on management strategies.
The development of new biologic agents with immunomodulatory actions has increased the interest of both doctors and the pharmaceutical companies in conducting clinical trials in BS. However, the lack of uniform, widely accepted outcome measures is an obstacle to designing randomized controlled trials (RCT) that meet regulatory agencies’ expectations. This in turn reduces the enthusiasm of industry support for such research. OMERACT has guided the development of data-driven outcome measures in several diseases. The OMERACT Vasculitis Working Group has developed a core set of outcome measures for use in clinical trials of antineutrophil cytoplasmic antibody–associated vasculitis and is continuing work on outcome measures for large-vessel vasculitis4,5. The Working Group is pursuing a similar approach, within the OMERACT framework, to develop validated outcome measures for clinical trials in BS.
As a first step in the development of a core set for BS, the aim of this systematic review is to describe the strengths and shortcomings of the outcomes and outcome measures that have been used in RCT, uncontrolled interventional studies, observational studies, longitudinal cohorts, case control studies, and biomarker and genetic association studies in BS.
METHODS
A systematic literature search was performed to identify all published articles that included defined outcome measures or outcomes in BS. PubMed was searched for articles published between January 1946 and November 2012. To avoid missing any relevant articles, no limits were used during the literature search, and titles and abstracts of all articles retrieved by the keywords “Behcet's syndrome OR Behcet's disease OR Adamantiades Behcet OR Behcet*” were evaluated for inclusion criteria.
Publications reviewed included all RCT, uncontrolled observational or retrospective interventional studies, longitudinal or retrospective cohort studies, case-control studies, biomarker studies, or genetic association studies reporting on at least 20 patients with BS and including at least 1 outcome or outcome measure. Original articles involving humans, published in English, German, French, or Turkish were included. A hand search of references of selected articles for identifying relevant studies was also performed. Unpublished reports, congress proceedings, or abstracts were excluded.
From the selected articles, data were extracted regarding the outcome measures and outcomes used in interventional studies and cohorts, as well as definitions of activity and severity used in biomarker and genetic association studies and case control studies. The outcomes and outcome measures were analyzed in 3 groups based on study type: (1) randomized controlled studies; (2) biomarker and genetic association studies; and (3) all other studies.
We also evaluated the outcomes and outcome measures in terms of their appropriateness to the OMERACT 2.0 Filter. We identified outcomes that addressed at least 1 of the 4 core areas incorporated into Filter 2.0: “death”, “life impact”, “pathophysiological manifestations”, and “resource use/economical impact area.”6
Quality assessment of the articles was not done because the aim of the exercise was to determine all outcomes and outcome measures used in trials of BS regardless of the methodological quality.
RESULTS
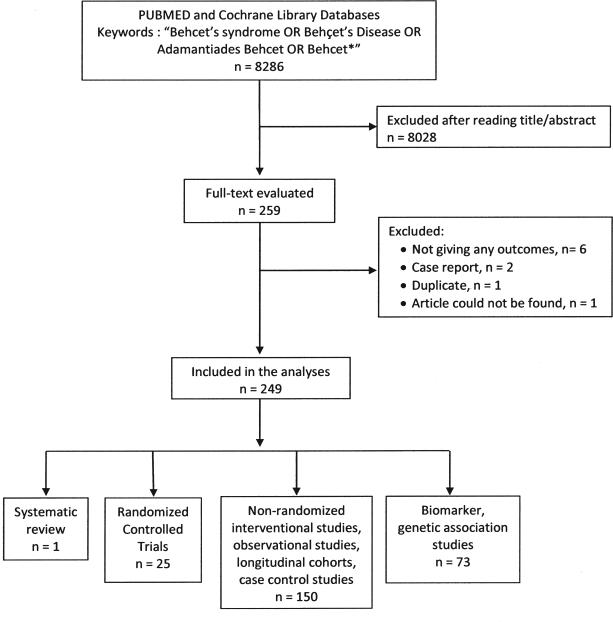
Figure 1 shows the flowchart of the systematic review results. In the first phase of the review, 8286 articles were identified. The following types of articles were excluded: informative reviews; case reports and other studies reporting on fewer than 20 patients; trials in languages other than English, French, German, or Turkish; clinical trials not reporting any outcomes such as epidemiological studies on the prevalence and types of involvement of BS in a country; trials not related to BS or where the primary area of interest was not BS such as general uveitis trials; and animal studies. Basic science studies including genetic association studies were excluded after reading the title and abstract. The full text of the remaining 259 articles were retrieved. Among these, 249 articles were ultimately determined to meet the full set of inclusion criteria. Tables 1–3 show the outcome measures used in these trials7-256.
Figure 1.
Outcomes and outcome measures used in studies of Behçet syndrome: systematic literature search strategy and results.
Table 1.
Overall disease assessment outcomes and outcome measures used in studies of Behçet syndrome. Numerals in parentheses indicate references.
| RCT*, n = 25 | Other**, n = 150 | Biomarker, n = 73 | Overall, n = 248 | |
|---|---|---|---|---|
| Behçet's Disease Current Activity Form (BDCAF) (7-30) | – | 17 | 7 | 24 |
| Clinical Disease Activity Index (32) | – | 1 | – | 1 |
| Clinical Manifestations Index (33,34,35) | 1 | 2 | – | 3 |
| Iranian BD Dynamic Activity Measure (18,20,31,37) | 1 | 3 | – | 4 |
| 1994 Criteria for Disease Activity of BD (36) | – | 1 | – | 1 |
| Activity, self-defined (38-87) | – | 4 | 46 | 50 |
| Activity, not defined (88-91) | – | 4 | 4 | |
| Krause's total severity score (38,46,51,57,104-114) | – | 11 | 4 | 15 |
| Severity (self-defined) (12,32,60,65,82,115-117) | 1 | 2 | 6 | 9 |
| Relapse (30,119-121) | – | 2 | 1 | 3 |
| Response (complete/partial) (30,122,123) | – | 3 | 3 | |
| Remission (complete/partial) (71,72,90,119-121) | 1 | 1 | 4 | 6 |
| Physicians Global Assessment (18,211) | – | 2 | – | 2 |
| BD Quality of Life (19,124,125) | – | 3 | – | 3 |
| Proportion of patients remaining on treatment (30) | – | 1 | – | 1 |
| Development of major organ involvement (98,122,174) | 1 | 2 | – | 3 |
| Death (92-103) | – | 11 | – | 11 |
Only 4 of the 25 RCT used an outcome measure for overall disease assessment.
The outcome measures used in uncontrolled interventional studies, cohorts, and case control studies are given together under the “other studies” column. A total of 66 outcome measures for overall disease assessment were used in these 150 studies. Similarly, biomarker studies and generic association studies are given together under the “Biomarker” column. RCT: randomized controlled trial; BD: Behçet disease.
Table 3.
Nondisease-specific outcome measures used in studies of Behçet syndrome. Numerals in parentheses indicate references.
| RCT, n = 0 | Other, n = 36 | Biomarker, n = 0 | Overall, n = 36 | |
|---|---|---|---|---|
| SF-36 (46,57,151,239) | – | 4 | – | 4 |
| Health-related QOL (224,239) | – | 2 | – | 2 |
| WHO QOL 100 (46) | – | 1 | – | 1 |
| WHO QOL BREF (240) | – | 1 | – | 1 |
| EQ-5D (229) | – | 1 | – | 1 |
| Dermatology Life Quality Index (12,255) | – | 2 | – | 2 |
| Oral Health Related Quality of Life (57) | – | 1 | – | 1 |
| Oral Health Impact Profile (57,128,131,234) | – | 4 | – | 4 |
| Nottingham Health Profile (23,141) | – | 2 | – | 2 |
| Life Satisfaction Index (23) | – | 1 | – | 1 |
| Lawton Instrumental Activities of Daily Living (125) | – | 1 | – | 1 |
| Kate Index of Activities of Daily Living (125) | – | 1 | – | 1 |
| Health Assessment Questionnaire (HAQ) (124,141) | – | 2 | – | 2 |
| Multidimensional HAQ (MDHAQ) (237,256) | – | 2 | – | 2 |
| Brief Symptom Inventory (230) | – | 1 | – | 1 |
| Beck Depression Scale (17,60,141,208,230,232,235,236,238,239,240) | – | 11 | – | 11 |
| Beck Anxiety Scale (230,238,239,241) | – | 4 | – | 4 |
| Beck Hopelessness Scale (238) | – | 1 | – | 1 |
| Hamilton Depression Rating Scale (12,228,241) | – | 3 | – | 3 |
| Hamilton Anxiety Rating Scale (12,228) | – | 2 | – | 2 |
| Center for Epidemiologic Studies Depression Scale (19) | – | 1 | – | 1 |
| Psychological General Wellbeing Scale (124,207) | – | 2 | – | 2 |
| Automatic Thoughts Questionnaire (238) | – | 1 | – | 1 |
| Hospital Depression Scale (231) | – | 1 | – | 1 |
| Hospital Anxiety Scale (231) | – | 1 | – | 1 |
| State Trait Anxiety Inventory (60,141) | – | 2 | – | 2 |
| Toronto Alexithymia Scale (241) | – | 1 | – | 1 |
| Epworth Sleepiness Scale (12) | – | 1 | – | 1 |
| Respiratory Disturbance Index (12) | – | 1 | – | 1 |
| Apneahypopnea Index (12) | – | 1 | – | 1 |
| Pittsburgh Sleep Quality Index (12,207) | – | 2 | – | 2 |
| Female Sexual Function Index (236) | – | 1 | – | 1 |
| International Index of Erectile Function (235) | – | 1 | – | 1 |
| Arizona Sexual Experience Scale (228) | – | 1 | – | 1 |
| Golombok Rust Sexual Satisfaction Scale (228) | – | 1 | – | 1 |
| Voice Handicap Test (233) | – | 1 | – | 1 |
| Fibromyalgia Impact Questionnaire (10,60) | – | 2 | – | 2 |
| Fatigue Severity Scale (12) | – | 1 | – | 1 |
SF-36: Medical Outcomes Study Short Form-36; WHO: World Health Organization; QOL: quality of life.
Outcome measures for overall disease assessment
The outcomes and outcome measures that are used for the overall evaluation of BS without organ-specific endpoints are given in Table 1. The most commonly used measure for evaluating disease activity was the Behçet's Disease Current Activity Form, which was used in 24/248 (9.6%) of the published studies7-30. There are 4 other activity measures that were used in a few trials each18,20,31-37. Several researchers used their own definition of activity38-87. The definitions of activity varied from requiring a minimum of 1 to 3 manifestations, including oral ulcers, genital ulcers, skin lesions, uveitis, vascular lesions, or neurologic lesions. Some studies did not provide an explanation of how active patients were defined88,89,90,91. Mortality is rarely used as a primary or even secondary endpoint of interest in studies of BS92-103.
For evaluating disease severity, the most commonly used index was the Krause Total Severity Score. This was used in 15/248 (6.0%) of published studies38,46,51,57,104-114. Several researchers used their own definition of disease severity12,32,60,65,82,115,116,117. Similarly, many studies incorporated outcomes unique to that study for definitions of relapse, response (complete/partial), and remission (complete/partial)30,71,72,90,118,119,120,121,122,123.
There was 1 validated health-related QOL measure specific for BS: the Behçet Disease Quality of Life. This measure has been used in only a few clinical trials19,124,125.
Organ-specific outcomes and outcome measures
Organ-specific outcomes and outcome measures used in trials are summarized in Table 2. The only organ-specific outcome measure that was developed for mucocutaneous BS and validated was the oral ulcer composite index126. The frequency, duration, size, pain, severity, and healing time of each of the mucocutaneous lesions were used as outcomes in different combinations in several studies57,118,120,127-135,138-140,185,243-251. Similarly, the number, frequency, severity, duration, and pain of arthritis episodes were reported in several studies132,133,137,139,141,142,143.
Table 2.
Organ specific outcomes and outcome measures used in studies of Behçet syndrome for mucocutaneous, musculoskeletal, eye, vascular, neurologic, and gastrointestinal involvement. Numerals in parentheses indicate references.
| RCT | Other* | Biomarker | Overall | |
|---|---|---|---|---|
| Mucocutaneous disease (n) | 18 | 14 | 0 | 32 |
| Frequency of oral ulcers/genital ulcers/nodular lesions/papulopustular lesions (57,120,131,135,138,139,243,245) | 6 | 2 | – | 8 |
| Number of oral ulcers/genital ulcers/nodular lesions/papulopustular lesions (127,128,130,132-135,137,139,140,185,244,246-248) | 10 | 5 | – | 15 |
| Duration of oral ulcers/genital ulcers/nodular lesions/papulopustular lesions (118,120,135,138) | 3 | 1 | – | 4 |
| Size of oral ulcers/genital ulcers (129,130,135,244,249) | 3 | 2 | – | 5 |
| Pain of oral ulcers/genital ulcers (120,129-131,134,243) | 4 | 2 | – | 6 |
| Severity of oral ulcers/genital ulcers (118,246) | 1 | 1 | – | 2 |
| Healing time of oral ulcers/genital ulcers (57,128-131,243,249) | 2 | 5 | – | 7 |
| Depth of oral ulcers/genital ulcers (244) | 1 | – | 1 | |
| Pathergy positivity (35,133,244) | 1 | 2 | – | 3 |
| Oral Ulcer Activity Index (126) | 1 | – | 1 | |
| Response (complete/partial) (30,118,136,137,250) | 3 | 2 | – | 5 |
| Remission (complete/partial) (251) | – | 1 | – | 1 |
| Musculoskeletal disease (n) | 6 | 1 | – | 7 |
| No. arthritis episodes (132,133,137,139,142) | 5 | – | 5 | |
| Frequency of arthritis episodes (139,141) | 1 | 1 | – | 2 |
| Severity of arthritis episodes (142) | 1 | – | – | 1 |
| Duration of arthritis episodes (139,142,143) | 3 | – | – | 3 |
| Tender joint score (143) | 1 | – | – | 1 |
| Degree of joint swelling (143) | 1 | – | – | 1 |
| Arthritis pain visual analog scale (143) | 1 | – | – | 1 |
| Vascular (n) | – | 14 | – | 14 |
| Venous thrombosis relapse (92,95,197) | – | 3 | – | 3 |
| New/recurrent aneurysm (96,100,198,199,201,203) | – | 6 | – | 6 |
| Disappearance of intracardiac thrombus (202) | – | 1 | – | 1 |
| Remission (93,94) | – | 2 | – | 2 |
| Death (92-96,100,102,199) | – | 8 | – | 8 |
| Amputation (96) | – | 1 | – | 1 |
| Operation need (95) | – | 1 | – | 1 |
| Postoperative complications (96,100,199,200,203) | – | 5 | – | 5 |
| Graft occlusion/patency (96,100,199,200,201,203) | – | 6 | – | 6 |
| Neurologic (n) | – | 7 | – | 8 |
| Functional outcome (independent/dependent/death) (206,210) | – | 2 | – | 2 |
| Improvement (97,204) | – | 2 | – | 2 |
| Poor outcome (97) | – | 1 | – | 1 |
| Death (97) | – | 1 | – | 1 |
| Relapse (204,205) | – | 2 | – | 2 |
| Progressive course (205) | – | 1 | – | 1 |
| Expanded Disability Status Scale (205,208,209) | – | 3 | – | 3 |
| Neuropathic pain (207) | – | 1 | – | 1 |
| Multiple sclerosis functional compound scale (208) | – | 1 | – | 1 |
| Eye involvement (n) | 7 | 53 | 3 | 63 |
| Ocular inflammatory attack (9,15,120,144,147,155-158,161,162,164-166,169-171,173,176,181,183,184,186,188-196) | 6 | 25 | 1 | 32 |
| Visual acuity (9,15,16,120,144-190) | 7 | 43 | 1 | 51 |
| Loss of useful vision/blindness (99,160,163,167,174-177) | – | 8 | – | 8 |
| Improvement of uveoretinitis (145) | – | 1 | – | 1 |
| Retinal vasculitis (149,166,181,185) | 2 | 1 | 1 | 4 |
| Hypopyon (149,166,181,185) | 2 | 1 | 1 | 4 |
| Ben Ezra Disease Activity Index (15,25,152,153) | 1 | 3 | – | 4 |
| Total Inflammatory Activity Index (152,153) | – | 2 | – | 2 |
| Total Adjusted Disease Act Index (152,153) | – | 2 | – | 2 |
| Worsening of intraocular inflammation (190) | 1 | – | 1 | |
| Regression of inflammatory signs (252) | – | 1 | – | 1 |
| Improvement in fundus fluorescein angiography findings (168) | 1 | – | – | 1 |
| Macular thickness (146,181) | 1 | 1 | – | 2 |
| Remission (16,155,157,163,165,169,186,193) | – | 7 | 1 | 8 |
| Response (complete/partial) (155,161) | – | 2 | – | 2 |
| Time to remission (191) | – | 1 | – | 1 |
| Time to relapse/recurrence (15,191,194) | – | 3 | – | 3 |
| National Eye Institute Visual Functioning Questionnaire (151) | – | 1 | – | 1 |
| Number of relapse-free patients (147) | – | 1 | – | 1 |
| Inflammation score – self-defined (159) | – | 1 | – | 1 |
| Duration of ocular attacks (171,188) | – | 2 | – | 2 |
| Vascular sheathing (181) | – | 1 | – | 1 |
| Venous occlusion (181) | – | 1 | – | 1 |
| Vitreous condensation (181) | – | 1 | – | 1 |
| SUN visual loss (16) | – | 1 | – | 1 |
| SUN lowering glucocorticoid dose to < 10 mg/day (253) | – | 1 | – | 1 |
| SUN control of uveitis with quiescence during maintenance (150) | – | 1 | – | 1 |
| SUN ocular relapse per patient year (150) | – | 1 | – | 1 |
| SUN intraocular inflammation (146,154,176) | – | 3 | – | 3 |
| SUN level of steroid dependence (154) | – | 1 | – | 1 |
| SUN complete/partial/no response (154) | – | 1 | – | 1 |
| SUN improvement/worsening (15,176) | – | 2 | – | 2 |
| Hogan's ocular inflammatory attack criteria for anterior uveitis (180,188,254) | – | 3 | – | 3 |
| Kimura's ocular inflammatory attack criteria for posterior uveitis (180,188) | – | 2 | – | 2 |
| Development of new eye disease (174,185) | 1 | 1 | – | 2 |
| Gastrointestinal (n) | – | 14 | 3 | 17 |
| Disease Activity Index for Intestinal BD (211,216,218,220,221) | – | 4 | 1 | 5 |
| Inflammatory Bowel Disease Questionnaire (224) | – | 1 | – | 1 |
| Crohn's Disease Activity Index (211,224,227) | – | 3 | – | 3 |
| Harvey Bradshaw Index for Activity (224,227) | – | 2 | – | 2 |
| St. Mark's Activity Index (224) | – | 1 | – | 1 |
| Relapse/recurrence (212-215,217,219,222,223,225,226) | – | 9 | 1 | 10 |
| Remission (212-214,223) | – | 3 | 1 | 4 |
| Operation need (211,212,214,217,221,223) | – | 5 | 1 | 6 |
| Reoperation need (213,217,219,222,225,226) | – | 6 | 6 | |
| Physician's global assessment (211) | – | 1 | 1 | |
| Immunosuppressive need (217,221) | – | 2 | 2 | |
| Glucocorticoid requirement (217,221) | – | 2 | 2 |
The outcome measures used in uncontrolled interventional studies, cohorts, and case control studies are given together under the “Other Studies” column. Similarly, biomarker studies and genetic association studies are given together under the “Biomarker” column. RCT: randomized controlled trial; BD: Behçet disease; SUN: Standardization of Uveitis Nomenclature.
Organ-specific outcome measures used in trials for eye involvement are given in Table 2. Visual acuity9,15,16,120,144-196,252-254 and inflammatory attacks9,15,120,144,147,155-158,161,162,164,165,166,169,170,171,173,176,181,183,184,186,188-196 are the most widely used outcome measures. However, the definition of an inflammatory flare varied widely among studies, and visual acuity was reported in several different ways, including change on Snellen chart or Japanese standard Landolt visual acuity chart, calculating the LogMar (logarithm of the minimum angle of resolution), or the percentage of patients who had a certain level of improvement. Loss of useful vision is another similar outcome used99,160,163,167,174,175,176,177. Different components of the Standardization of Uveitis Nomenclature Working Group Criteria, a generic uveitis measure, were used in some of the trials for BS14-16,146,150,154,176. The Ben Ezra Disease Activity Index, specifically developed for evaluating uveitis in BS, was also used in a few studies15,25,152,153.
Outcomes and outcome measures used in trials for vascular 92,93,94,95,96,100,102,197-203, neurologic97,204-210, and GI involvement211-227 are given in Table 2. There were no RCT or prospective interventional trials for vascular involvement of BS. Studies that report on longterm followup of patients with BS with vascular involvement or retrospective reviews of surgical outcomes usually reported relapses/recurrences, remission, operation and reoperation rates and postoperative complications92,93,94,95,96,100,102,197-203. Relapses/recurrences were defined as a new lesion or a progression of an already present lesion. Remission was defined as the absence of a new or progressing lesion.
There were also no RCT for neurologic or GI involvement of BS. There is 1 GI index that was developed and validated specifically for BS (Disease Activity Index for Intestinal Behçet Disease)211,216,218,220,221. This index showed a higher correlation with physician global assessment and higher responsiveness than the Crohn Disease Activity Index. Many studies used indexes and outcomes developed for inflammatory bowel diseases211,224,227.
Laboratory outcome measures
Erythrocyte sedimentation rate9,16,22,24,34,40,43,47,49,59,60,61,63,66,67,68,69,76,77,85,89,94,139,143,177,220,228 and C-reactive protein9,16,24,40,43,47,49,59,60,66,67,76,77,85,89,94,143,177,216,220,228 levels were used as indicators of disease activity. No laboratory measure has been validated as a biomarker of disease activity in BS.
Generic measures
Several generic measures were used to evaluate the QOL and psychological, cognitive, or sexual effect of BS (Table 3)10,17,19,23,46,57,60,124,125,128,141,151,207,208,228-241,255,256. Among these, only the oral health-related QOL was specifically validated for BS128. The most frequently used psychological indexes were the Beck Depression Index17,60,141,208,230,232,235,236,238,239,240 and Beck Anxiety Scale230,238,239,241.
Evaluation of the outcomes that were reviewed according to the OMERACT 2.0 Filter
Death, which is one of the core areas in the Filter 2.0, was included as an outcome in 12 trials92-103. None of the RCT reported death. Life impact was addressed by 1 BS specific measure, Behçet Disease Quality of Life19,124,125, and several generic measures given in Table 3 10,17,19,23,46,57,60,124,125,128,141,151,207,208,228-241,255,256. There was 1 cost analysis study in BS that retrospectively analyzed direct costs such as medication, diagnostic tests, hospital visits, hospitalization fees, and lodging and transportation expenses and indirect costs such as lost workdays and wages by questioning the patients242. However, none of the interventional trials included an instrument to address the “resource use/economical impact area.” The rest of the outcomes given in Table 1 and 2 are related to the “patho-physiological manifestations” area.
DISCUSSION
This systematic review revealed the diversity and variability in the outcomes and outcome measures used in clinical research in BS. It shows that there are no standard definitions for most outcomes and few validated outcome tools for any aspect of disease assessment in BS. The number of different outcomes assessed and the inconsistency in assessment methods is problematic. For example, visual acuity was used in 51 of the 63 trials studying eye disease in BS, but 5 different assessment methods were used among the 51 trials, making comparison of trial results extremely difficult.
There are 5 activity scales, 1 severity scale, a composite index for oral ulcers, and a QOL scale that were developed for BS. However, these measures have not been widely adopted for use by clinical researchers. Many investigators have preferred to use their own definitions of activity38-87 or severity12,32,60,65,82,115,116,117, or have included “generic” measures such as the Medical Outcomes Study Short Form-36 in their trials46,57,151,239. Similarly, some authors studying specific physiologic manifestations of BS have used outcome tools developed for other diseases, for example, uveitis scales or measures of inflammatory bowel disease; however, these tools have not been validated for BS. It may be appropriate to adapt existing tools for use in BS, but such use should be supported by properly conducted data analyses. Regarding the disease specific scores, they are composite scores of each item such as oral ulcers, genital ulcers, skin lesions, and eye lesions, and were not developed with the purpose of evaluating the individual items separately; however, it would be interesting to study the performance of each item outside composite scores and compare them to the organ-specific outcome measures.
During the OMERACT 11 meeting, the new Filter 2.0 was introduced. The Filter 2.0 approach suggests that all core sets of outcome measures should include at least 1 instrument from the 3 core areas of “death”, “life impact”, and “pathophysiological manifestations”, and preferably 1 outcome measure addressing “resource use/economical impact area.” Thus, we evaluated the outcomes and outcome measures used in BS trials in terms of their relevance to these core areas. None of the RCT reported death. It can be assumed that there were no deaths since it was not mentioned among the adverse events; however, death was not formally reported in any of the trials. Similarly “resource use/economical impact area” was not covered in any of the interventional trials. The outcomes and outcome measures used were mostly related to the “life impact” and “pathophysiological manifestations” areas. Work on the development of a core set of outcomes for BS should strive to address each of the core areas of Filter 2.0.
Part of the evolving research agenda for the OMERACT Vasculitis Working Group is to work toward developing a core set of outcomes in BS. Among the next steps in this process is to conduct a Delphi exercise among international investigators and clinical experts in BS to reach consensus on outcomes of interest in BS. Apart from determining the current status of outcomes research in BS, the data derived from this systematic literature review will be used as a starting point to identify the domains and candidate instruments for use in the Delphi exercise. The collaboration of investigators from many different regions of the world and from different medical specialties, including rheumatology, ophthalmology, dermatology, gastroenterology, and neurology will be needed to develop a core set of outcome measures that are properly tested, well validated, and broadly acceptable for use in randomized trials in BS.
Acknowledgments
Supported by the Vasculitis Clinical Research Consortium (www.RareDiseasesNetwork.org/vcrc) through the US National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319), the National Center for Research Resources (U54 RR01949703), the Office of Rare Diseases Research/National Center for Advancing Translational Sciences, and the Patient-Centered Outcomes Research Institute.
Contributor Information
Gulen Hatemi, Division of Rheumatology, Department of Internal Medicine, Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey.
Peter A. Merkel, Division of Rheumatology and Department of Epidemiology, University of Pennsylvania, Philadelphia, PA, USA.
Vedat Hamuryudan, Division of Rheumatology, Department of Internal Medicine, Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey.
Maarten Boers, Department of Clinical Epidemiology, VU University Medical Center, Amsterdam, The Netherlands.
Haner Direskeneli, Department of Rheumatology, Marmara University School of Medicine, Istanbul, Turkey.
Sibel Z. Aydin, Department of Rheumatology, Istanbul Medeniyet University, Goztepe Training and Research Hospital, Istanbul, Turkey.
Hasan Yazici, Division of Rheumatology, Department of Internal Medicine, Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey..
REFERENCES
- 1.Yazici H, Ugurlu S, Seyahi E. Behcet syndrome: is it one condition? Clin Rev Allergy Immunol. 2012;43:275–80. doi: 10.1007/s12016-012-8319-x. [DOI] [PubMed] [Google Scholar]
- 2.Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, et al. EULAR recommendations for the management of Behcet disease. Ann Rheum Dis. 2008;67:1656–62. doi: 10.1136/ard.2007.080432. [DOI] [PubMed] [Google Scholar]
- 3.Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A, et al. Management of Behcet disease: a systematic literature review for the European League Against Rheumatism evidence-based recommendations for the management of Behcet disease. Ann Rheum Dis. 2009;68:1528–34. doi: 10.1136/ard.2008.087957. [DOI] [PubMed] [Google Scholar]
- 4.Direskeneli H, Aydin SZ, Kermani TA, Matteson EL, Boers M, Herlyn K, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol. 2011;38:1471–9. doi: 10.3899/jrheum.110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merkel PA, Aydin SZ, Boers M, Direskeneli H, Herlyn K, Seo P, et al. The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J Rheumatol. 2011;38:1480–6. doi: 10.3899/jrheum.110276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boers M, Kirwan J, Gossec L, Conaghan P, D'Agostino M-A, Bingham C, III, et al. How to choose core outcome sets for clinical trials: OMERACT 11 approves Filter 2.0. J Rheumatol. 2014:41. doi: 10.3899/jrheum.131314. in press. [DOI] [PubMed] [Google Scholar]
- 7.Gheita TA, Raafat H, Khalil H, Hussein H. Serum level of APRIL/BLyS in Behcet's disease patients: clinical significance in uveitis and disease activity. Mod Rheumatol. 2013;23:542–6. doi: 10.1007/s10165-012-0694-x. [DOI] [PubMed] [Google Scholar]
- 8.Gheita TA, Samir H, Hussein H. Anti-annexin V antibodies in neuro-Behcet patients: clinical significance and relation to disease activity. Int J Rheum Dis. 2012;15:e124–6. doi: 10.1111/j.1756-185X.2012.01759.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida A, Kaburaki T, Okinaga K, Takamoto M, Kawashima H, Fujino Y. Clinical background comparison of patients with and without ocular inflammatory attacks after initiation of infliximab therapy. Jpn J Ophthalmol. 2012;56:536–43. doi: 10.1007/s10384-012-0182-z. [DOI] [PubMed] [Google Scholar]
- 10.Melikoglu M, Melikoglu MA. The prevalence of fibromyalgia in patients with Behcet's disease and its relation with disease activity. Rheumatol Int. 2013;33:1219–22. doi: 10.1007/s00296-012-2530-1. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed RH, Nasef A, Kewan HH, Al Shaar M. Vascular neurobehcet disease: correlation with current disease activity forum and systemic vascular involvement. Clin Rheumatol. 2012;31:1033–40. doi: 10.1007/s10067-012-1953-0. [DOI] [PubMed] [Google Scholar]
- 12.Tascilar NF, Tekin NS, Ankarali H, Sezer T, Atik L, Emre U, et al. Sleep disorders in Behcet's disease, and their relationship with fatigue and quality of life. J Sleep Res. 2012;21:281–8. doi: 10.1111/j.1365-2869.2011.00976.x. [DOI] [PubMed] [Google Scholar]
- 13.Han EC, Cho SB, Ahn KJ, Oh SH, Kim J, Kim DS, et al. Expression of pro-inflammatory protein S100A12 (EN-RAGE) in Behcet's disease and its association with disease activity: a pilot study. Ann Dermatol. 2011;23:313–20. doi: 10.5021/ad.2011.23.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozden MG, Cayci YT, Tekin H, Coban AY, Aydin F, Senturk N, et al. Serum galectin-3 levels in patients with Behcet's disease: association with disease activity over a long-term follow-up. J Eur Acad Dermatol Venereol. 2011;25:1168–73. doi: 10.1111/j.1468-3083.2010.03941.x. [DOI] [PubMed] [Google Scholar]
- 15.Deuter CM, Zierhut M, Mohle A, Vonthein R, Stobiger N, Kotter I. Long-term remission after cessation of interferon-alpha treatment in patients with severe uveitis due to Behcet's disease. Arthritis Rheum. 2010;62:2796–805. doi: 10.1002/art.27581. [DOI] [PubMed] [Google Scholar]
- 16.Giardina A, Ferrante A, Ciccia F, Vadala M, Giardina E, Triolo G. One year study of efficacy and safety of infliximab in the treatment of patients with ocular and neurological Behcet's disease refractory to standard immunosuppressive drugs. Rheumatol Int. 2011;31:33–7. doi: 10.1007/s00296-009-1213-z. [DOI] [PubMed] [Google Scholar]
- 17.Melikoglu MA, Melikoglu M. The relationship between disease activity and depression in patients with Behcet disease and rheumatoid arthritis. Rheumatol Int. 2010;30:941–6. doi: 10.1007/s00296-009-1080-7. [DOI] [PubMed] [Google Scholar]
- 18.Shahram F, Khabbazi A, Nadji A, Ziaie N, Banihashemi AT, Davatchi F. Comparison of existing disease activity indices in the follow-up of patients with Behcet's disease. Mod Rheumatol. 2009;19:536–41. doi: 10.1007/s10165-009-0191-z. [DOI] [PubMed] [Google Scholar]
- 19.Yi SW, Kim JH, Lim KY, Bang D, Lee S, Lee ES. The Behcet's Disease Quality of Life: reliability and validity of the Korean version. Yonsei Med J. 2008;49:698–704. doi: 10.3349/ymj.2008.49.5.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simsek I, Meric C, Erdem H, Pay S, Kilic S, Dinc A. Accuracy of recall of the items included in disease activity forms of Behcet's disease: comparison of retrospective questionnaires with a daily telephone interview. Clin Rheumatol. 2008;27:1255–60. doi: 10.1007/s10067-008-0899-8. [DOI] [PubMed] [Google Scholar]
- 21.Neves FS, Moraes JC, Kowalski SC, Goldenstein-Schainberg C, Lage LV, Goncalves CR. Cross-cultural adaptation of the Behcet's Disease Current Activity Form (BDCAF) to Brazilian Portuguese language. Clin Rheumatol. 2007;26:1263–7. doi: 10.1007/s10067-006-0484-y. [DOI] [PubMed] [Google Scholar]
- 22.Kwon SR, Lim MJ, Park SG, Moon YS, Park W. Decreased protein S activity is related to the disease activity of Behcet's disease. Rheumatol Int. 2006;27:39–43. doi: 10.1007/s00296-006-0214-4. [DOI] [PubMed] [Google Scholar]
- 23.Bodur H, Borman P, Ozdemir Y, Atan C, Kural G. Quality of life and life satisfaction in patients with Behcet's disease: relationship with disease activity. Clin Rheumatol. 2006;25:329–33. doi: 10.1007/s10067-005-0046-8. [DOI] [PubMed] [Google Scholar]
- 24.Musabak U, Pay S, Erdem H, Simsek I, Pekel A, Dinc A, et al. Serum interleukin-18 levels in patients with Behcet's disease. Is its expression associated with disease activity or clinical presentations? Rheumatol Int. 2006;26:545–50. doi: 10.1007/s00296-005-0029-8. [DOI] [PubMed] [Google Scholar]
- 25.Kotter I, Vonthein R, Zierhut M, Eckstein AK, Ness T, Gunaydin I, et al. Differential efficacy of human recombinant interferon-alpha2a on ocular and extraocular manifestations of Behcet disease: results of an open 4-center trial. Semin Arthritis Rheum. 2004;33:311–9. doi: 10.1016/j.semarthrit.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Lawton G, Bhakta BB, Chamberlain MA, Tennant A. The Behcet's disease activity index. Rheumatology. 2004;43:73–8. doi: 10.1093/rheumatology/keg453. [DOI] [PubMed] [Google Scholar]
- 27.Akarsu M, Demirkan F, Ozsan GH, Onen F, Yuksel F, Ozkan S, et al. Increased levels of tissue factor pathway inhibitor may reflect disease activity and play a role in thrombotic tendency in Behcet's disease. Am J Hematol. 2001;68:225–30. doi: 10.1002/ajh.1186. [DOI] [PubMed] [Google Scholar]
- 28.Hamuryudan V, Fresko I, Direskeneli H, Tenant MJ, Yurdakul S, Akoglu T, et al. Evaluation of the Turkish translation of a disease activity form for Behcet's syndrome. Rheumatology. 1999;38:734–6. doi: 10.1093/rheumatology/38.8.734. [DOI] [PubMed] [Google Scholar]
- 29.Bhakta BB, Brennan P, James TE, Chamberlain MA, Noble BA, Silman AJ. Behcet's disease: evaluation of a new instrument to measure clinical activity. Rheumatology. 1999;38:728–33. doi: 10.1093/rheumatology/38.8.728. [DOI] [PubMed] [Google Scholar]
- 30.Furuta S, Chow Y, Chaudhry A, Jayne D. Switching of anti-TNF-α agents in Behçet's disease. Clin Exp Rheumatol. 2012;30(Suppl 72):62–8. [PubMed] [Google Scholar]
- 31.Davatchi F, Sadeghi Abdollahi B, Tehrani Banihashemi A, Shahram F, Nadji A, Shams H, et al. Colchicine versus placebo in Behcet's disease: randomized, double-blind, controlled crossover trial. Mod Rheumatol. 2009;19:542–9. doi: 10.1007/s10165-009-0200-2. [DOI] [PubMed] [Google Scholar]
- 32.Harzallah O, Kerkeni A, Baati T, Mahjoub S. Oxidative stress: correlation with Behcet's disease duration, activity and severity. Eur J Intern Med. 2008;19:541–7. doi: 10.1016/j.ejim.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Najim RA, Sharquie KE, Abu-Raghif AR. Oxidative stress in patients with Behcet's disease: I correlation with severity and clinical parameters. J Dermatol. 2007;34:308–14. doi: 10.1111/j.1346-8138.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 34.Sharquie KE, Najim RA, Al-Dori WS, Al-Hayani RK. Oral zinc sulfate in the treatment of Behcet's disease: a double blind cross-over study. J Dermatol. 2006;33:541–6. doi: 10.1111/j.1346-8138.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 35.Al-Waiz MM, Sharquie KE, A-Qaissi MH, Hayani RK. Colchicine and benzathine penicillin in the treatment of Behcet disease: a case comparative study. Dermatol Online J. 2005;11:3. [PubMed] [Google Scholar]
- 36.Triolo G, Accardo-Palumbo A, Dieli F, Ciccia F, Ferrante A, Giardina E, et al. Vgamma9/Vdelta2 T lymphocytes in Italian patients with Behcet's disease: evidence for expansion, and tumour necrosis factor receptor II and interleukin-12 receptor beta1 expression in active disease. Arthritis Res Ther. 2003;5:R262–8. doi: 10.1186/ar785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adler YD, Mansmann U, Zouboulis CC. Mycophenolate mofetil is ineffective in the treatment of mucocutaneous Adamantiades-Behcet's disease. Dermatology. 2001;203:322–4. doi: 10.1159/000051781. [DOI] [PubMed] [Google Scholar]
- 38.Bassyouni IH, El-Wakd MM, Bassyouni RH. Soluble levels of osteopontin in patients with Behcet's disease: association with disease activity and vascular involvement. J Clin Immunol. 2013;33:361–7. doi: 10.1007/s10875-012-9820-y. [DOI] [PubMed] [Google Scholar]
- 39.Mumcu G, Cimilli H, Karacayli U, Inanc N, Ture-Ozdemir F, Eksioglu-Demiralp E, et al. Salivary levels of HNP 1-3 are related to oral ulcer activity in Behcet's disease. Int J Dermatol. 2013;52:1198–201. doi: 10.1111/j.1365-4632.2012.05504.x. [DOI] [PubMed] [Google Scholar]
- 40.Orem A, Yayli S, Arica DA, Akcan B, Yucesan FB, Bahadir S. Lipoprotein-associated phospholipase A(2) level in patients with Behcet's disease. J Eur Acad Dermatol Venereol. 2012 Jul 3; doi: 10.1111/j.1468-3083.2012.04631.x. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 41.Cimen F, Yildirmak ST, Ergen A, Cakmak M, Dogan S, Yenice N, et al. Serum lipid, lipoprotein and oxidatively modified low density lipoprotein levels in active or inactive patients with Behcet's disease. Indian J Dermatol. 2012;57:97–101. doi: 10.4103/0019-5154.94273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kato Y, Yamamoto T. Serum levels of GRO-alpha are elevated in association with disease activity in patients with Behcet's disease. Int J Dermatol. 2012;51:286–9. doi: 10.1111/j.1365-4632.2011.04998.x. [DOI] [PubMed] [Google Scholar]
- 43.Sahin E, Karaman G, Uslu M, Karul A, Sendur N, Savk E. Adiponectin levels, insulin resistance and their relationship with serum levels of inflammatory cytokines in patients with Behcet's disease. J Eur Acad Dermatol Venereol. 2012;26:1498–502. doi: 10.1111/j.1468-3083.2011.04318.x. [DOI] [PubMed] [Google Scholar]
- 44.Ekinci NS, Alpsoy E, Karakas AA, Yilmaz SB, Yegin O. IL-17A has an important role in the acute attacks of Behcet's disease. J Invest Dermatol. 2010;130:2136–8. doi: 10.1038/jid.2010.114. [DOI] [PubMed] [Google Scholar]
- 45.Fadini GP, Tognon S, Rodriguez L, Boscaro E, Baesso I, Avogaro A, et al. Low levels of endothelial progenitor cells correlate with disease duration and activity in patients with Behcet's disease. Clin Exp Rheumatol. 2009;27:814–21. [PubMed] [Google Scholar]
- 46.Ertam I, Kitapcioglu G, Aksu K, Keser G, Ozaksar A, Elbi H, et al. Quality of life and its relation with disease severity in Behcet's disease. Clin Exp Rheumatol. 2009;27(Suppl 53):S18–22. [PubMed] [Google Scholar]
- 47.Erturan I, Basak PY, Ozturk O, Ceyhan AM, Akkaya VB. Is there any relationship between serum and urine neopterin and serum interferon-gamma levels in the activity of Behcet's disease? J Eur Acad Dermatol Venereol. 2009;23:1414–8. doi: 10.1111/j.1468-3083.2009.03334.x. [DOI] [PubMed] [Google Scholar]
- 48.Serarslan G, Sogut S, Yonden Z, Oksuz H, Savas N, Yenin JZ, et al. Increased macrophage migration inhibitory factor in Behcet's disease and relation with the disease activity. J Eur Acad Dermatol Venereol. 2009;23:1344–6. doi: 10.1111/j.1468-3083.2009.03223.x. [DOI] [PubMed] [Google Scholar]
- 49.Kutlay S, Calayoglu R, Boyvat A, Turkcapar N, Sengul S, Keven K, et al. Circulating endothelial cells: a disease activity marker in Behcet's vasculitis? Rheumatol Int. 2008;29:159–62. doi: 10.1007/s00296-008-0656-y. [DOI] [PubMed] [Google Scholar]
- 50.Turan B, Pfister K, Diener PA, Hell M, Moller B, Boyvat A, et al. Soluble tumour necrosis factor receptors sTNFR1 and sTNFR2 are produced at sites of inflammation and are markers of arthritis activity in Behcet's disease. Scand J Rheumatol. 2008;37:135–41. doi: 10.1080/03009740701747137. [DOI] [PubMed] [Google Scholar]
- 51.Lee YJ, Kang SW, Song JK, Park JJ, Bae YD, Lee EY, et al. Serum galectin-3 and galectin-3 binding protein levels in Behcet's disease and their association with disease activity. Clin Exp Rheumatol. 2007;25(Suppl 45):S41–5. [PubMed] [Google Scholar]
- 52.Protogerou AD, Sfikakis PP, Stamatelopoulos KS, Papamichael C, Aznaouridis K, Karatzis E, et al. Interrelated modulation of endothelial function in Behcet's disease by clinical activity and corticosteroid treatment. Arthritis Res Ther. 2007;9:R90. doi: 10.1186/ar2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gullu H, Caliskan M, Erdogan D, Yilmaz S, Dursun R, Ciftci O, et al. Patients with Behcet's disease carry a higher risk for microvascular involvement in active disease period. Ann Med. 2007;39:154–9. doi: 10.1080/07853890701204866. [DOI] [PubMed] [Google Scholar]
- 54.Sarican T, Ayabakan H, Turkmen S, Kalaslioglu V, Baran F, Yenice N. Homocysteine: an activity marker in Behcet's disease? J Dermatol Sci. 2007;45:121–6. doi: 10.1016/j.jdermsci.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Caliskan M, Yilmaz S, Yildirim E, Gullu H, Erdogan D, Ciftci O, et al. Endothelial functions are more severely impaired during active disease period in patients with Behcet's disease. Clin Rheumatol. 2007;26:1074–8. doi: 10.1007/s10067-006-0449-1. [DOI] [PubMed] [Google Scholar]
- 56.Aki T, Karincaoglu Y, Seyhan M, Batcioglu K. Serum substance P and calcitonin gene-related peptide levels in Behcet's disease and their association with disease activity. Clin Exp Dermatol. 2006;31:583–7. doi: 10.1111/j.1365-2230.2006.02157.x. [DOI] [PubMed] [Google Scholar]
- 57.Mumcu G, Inanc N, Ergun T, Ikiz K, Gunes M, Islek U, et al. Oral health related quality of life is affected by disease activity in Behcet's disease. Oral Dis. 2006;12:145–51. doi: 10.1111/j.1601-0825.2005.01173.x. [DOI] [PubMed] [Google Scholar]
- 58.Esmat S, El Sherif H, Anwar S, Fahmy I, Elmenyawi M, Shaker O. Lipoprotein (a) and nitrites in Behcet's disease: relationship with disease activity and vascular complications. Eur J Dermatol. 2006;16:67–71. [PubMed] [Google Scholar]
- 59.Coskun B, Saral Y, Godekmerdan A, Erden I, Coskun N. Activation markers in Behcet's disease. Skinmed. 2005;4:282–6. doi: 10.1111/j.1540-9740.2005.03865.x. [DOI] [PubMed] [Google Scholar]
- 60.Lee SS, Yoon HJ, Chang HK, Park KS. Fibromyalgia in Behcet's disease is associated with anxiety and depression, and not with disease activity. Clin Exp Rheumatol. 2005;23(Suppl 38):S15–9. [PubMed] [Google Scholar]
- 61.Turkoz Y, Evereklioglu C, Ozkiris A, Mistik S, Borlu M, Ozerol IH, et al. Serum levels of soluble P-selectin are increased and associated with disease activity in patients with Behcet's syndrome. Mediators Inflamm. 2005;2005:237–41. doi: 10.1155/MI.2005.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gur-Toy G, Lenk N, Yalcin B, Aksaray S, Alli N. Serum interleukin-8 as a serologic marker of activity in Behcet's disease. Int J Dermatol. 2005;44:657–60. doi: 10.1111/j.1365-4632.2004.02184.x. [DOI] [PubMed] [Google Scholar]
- 63.Duygulu F, Evereklioglu C, Calis M, Borlu M, Cekmen M, Ascioglu O. Synovial nitric oxide concentrations are increased and correlated with serum levels in patients with active Behcet's disease: a pilot study. Clin Rheumatol. 2005;24:324–30. doi: 10.1007/s10067-004-1015-3. [DOI] [PubMed] [Google Scholar]
- 64.Atasoy M, Karatay S, Yildirim K, Kadi M, Erdem T, Senel K. The relationship between serum prolactin levels and disease activity in patients with Behcet's disease. Cell Biochem Funct. 2006;24:353–6. doi: 10.1002/cbf.1227. [DOI] [PubMed] [Google Scholar]
- 65.Ates A, Kinikli G, Duzgun N, Duman M. Lack of association of tumor necrosis factor-alpha gene polymorphisms with disease susceptibility and severity in Behcet's disease. Rheumatol Int. 2006;26:348–53. doi: 10.1007/s00296-005-0610-1. [DOI] [PubMed] [Google Scholar]
- 66.Calis M, Ates F, Yazici C, Kose K, Kirnap M, Demir M, et al. Adenosine deaminase enzyme levels, their relation with disease activity, and the effect of colchicine on adenosine deaminase levels in patients with Behcet's disease. Rheumatol Int. 2005;25:452–6. doi: 10.1007/s00296-005-0612-z. [DOI] [PubMed] [Google Scholar]
- 67.Yazici C, Kose K, Calis M, DemIr M, Kirnap M, Ates F. Increased advanced oxidation protein products in Behcet's disease: a new activity marker? Br J Dermatol. 2004;151:105–11. doi: 10.1111/j.1365-2133.2004.06003.x. [DOI] [PubMed] [Google Scholar]
- 68.Evereklioglu C, Ozbek E, Cekmen M, Mehmet N, Duygulu F, Ozkiris A, et al. Urinary nitric oxide levels are increased and correlated with plasma concentrations in patients with Behcet's disease: is it a new urinary activity marker? Nephrology. 2003;8:231–8. doi: 10.1046/j.1440-1797.2003.00180.x. [DOI] [PubMed] [Google Scholar]
- 69.Cekmen M, Evereklioglu C, Er H, Inaloz HS, Doganay S, Turkoz Y, et al. Vascular endothelial growth factor levels are increased and associated with disease activity in patients with Behcet's syndrome. Int J Dermatol. 2003;42:870–5. doi: 10.1046/j.1365-4362.2003.01688.x. [DOI] [PubMed] [Google Scholar]
- 70.Sandikci R, Turkmen S, Guvenen G, Ayabakan H, Gulcan P, Koldas M, et al. Lipid peroxidation and antioxidant defence system in patients with active or inactive Behcet's disease. Acta Derm Venereol. 2003;83:342–6. doi: 10.1080/00015550310003782. [DOI] [PubMed] [Google Scholar]
- 71.Hamzaoui K, Hamzaoui A, Guemira F, Bessioud M, Hamza M, Ayed K. Cytokine profile in Behcet's disease patients. Relationship with disease activity. Scand J Rheumatol. 2002;31:205–10. doi: 10.1080/030097402320318387. [DOI] [PubMed] [Google Scholar]
- 72.Evereklioglu C, Inaloz HS, Kirtak N, Doganay S, Bulbul M, Ozerol E, et al. Serum leptin concentration is increased in patients with Behcet's syndrome and is correlated with disease activity. Br J Dermatol. 2002;147:331–6. doi: 10.1046/j.1365-2133.2002.04703.x. [DOI] [PubMed] [Google Scholar]
- 73.Aygunduz M, Bavbek N, Ozturk M, Kaftan O, Kosar A, Kirazli S. Serum beta 2-microglobulin reflects disease activity in Behcet's disease. Rheumatol Int. 2002;22:5–8. doi: 10.1007/s00296-002-0180-4. [DOI] [PubMed] [Google Scholar]
- 74.Odabas AR, Karakuzu A, Cetinkaya R, Selcuk Y, Keles S, Bilen H. Increased serum ferritin levels in active Behcet's disease. Int J Clin Pract. 2002;56:310–1. [PubMed] [Google Scholar]
- 75.Evereklioglu C, Turkoz Y, Er H, Inaloz HS, Ozbek E, Cekmen M. Increased nitric oxide production in patients with Behcet's disease: is it a new activity marker? J Am Acad Dermatol. 2002;46:50–4. doi: 10.1067/mjd.2002.118338. [DOI] [PubMed] [Google Scholar]
- 76.Gurbuz O, Ozdemir Y, Cosar CB, Kural G. Lipoprotein (a) in Behcet's disease as an indicator of disease activity and in thrombotic complications. Eur J Ophthalmol. 2001;11:62–5. doi: 10.1177/112067210101100112. [DOI] [PubMed] [Google Scholar]
- 77.Katsantonis J, Adler Y, Orfanos CE, Zouboulis CC. Adamantiades-Behcet's disease: serum IL-8 is a more reliable marker for disease activity than C-reactive protein and erythrocyte sedimentation rate. Dermatology. 2000;201:37–9. doi: 10.1159/000018426. [DOI] [PubMed] [Google Scholar]
- 78.Zouboulis CC, Katsantonis J, Ketteler R, Treudler R, Kaklamani E, Hornemann S, et al. Adamantiades-Behcet's disease: interleukin-8 is increased in serum of patients with active oral and neurological manifestations and is secreted by small vessel endothelial cells. Arch Dermatol Res. 2000;292:279–84. doi: 10.1007/s004030000128. [DOI] [PubMed] [Google Scholar]
- 79.Alpsoy E, Cayirli C, Er H, Yilmaz E. The levels of plasma interleukin-2 and soluble interleukin-2R in Behcet's disease: a marker of disease activity. J Dermatol. 1998;25:513–6. doi: 10.1111/j.1346-8138.1998.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 80.Sugi-Ikai N, Nakazawa M, Nakamura S, Ohno S, Minami M. Increased frequencies of interleukin-2- and interferon-gamma-producing T cells in patients with active Behcet's disease. Invest Ophthalmol Vis Sci. 1998;39:996–1004. [PubMed] [Google Scholar]
- 81.Turan B, Gallati H, Erdi H, Gurler A, Michel BA, Villiger PM. Systemic levels of the T cell regulatory cytokines IL-10 and IL-12 in Behcet's disease; soluble TNFR-75 as a biological marker of disease activity. J Rheumatol. 1997;24:128–32. [PubMed] [Google Scholar]
- 82.Yosipovitch G, Shohat B, Bshara J, Wysenbeek A, Weinberger A. Elevated serum interleukin 1 receptors and interleukin 1B in patients with Behcet's disease: correlations with disease activity and severity. Isr J Med Sci. 1995;31:345–8. [PubMed] [Google Scholar]
- 83.Aydintug AO, Tokgoz G, Ozoran K, Duzgun N, Gurler A, Tutkak H. Elevated levels of soluble intercellular adhesion molecule-1 correlate with disease activity in Behcet's disease. Rheumatol Int. 1995;15:75–8. doi: 10.1007/BF00262712. [DOI] [PubMed] [Google Scholar]
- 84.Cervera R, Navarro M, Lopez-Soto A, Cid MC, Font J, Esparza J, et al. Antibodies to endothelial cells in Behcet's disease: cell-binding heterogeneity and association with clinical activity. Ann Rheum Dis. 1994;53:265–7. doi: 10.1136/ard.53.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muftuoglu AU, Yazici H, Yurdakul S, Tuzun Y, Pazarli H, Gungen G, et al. Behcet's disease. Relation of serum C-reactive protein and erythrocyte sedimentation rates to disease activity. Int J Dermatol. 1986;25:235–9. doi: 10.1111/j.1365-4362.1986.tb02232.x. [DOI] [PubMed] [Google Scholar]
- 86.Yazici H, Tuzun Y, Pazarli H, Yurdakul S, Ozyazgan Y, Ozdogan H, et al. Influence of age of onset and patient's sex on the prevalence and severity of manifestations of Behcet's syndrome. Ann Rheum Dis. 1984;43:783–9. doi: 10.1136/ard.43.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yalcindag FN, Yalcindag A, Caglayan O, Ozdemir O. Serum haptoglobin levels in ocular Behcet disease and acute phase proteins in the course of Behcet disease. Eur J Ophthalmol. 2008;18:787–91. doi: 10.1177/112067210801800520. [DOI] [PubMed] [Google Scholar]
- 88.Lee JS, Park MJ, Park S, Lee ES. Differential expression of T cell immunoglobulin- and mucin-domain-containing molecule-3 (TIM-3) according to activity of Behcet's disease. J Dermatol Sci. 2012;65:220–2. doi: 10.1016/j.jdermsci.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 89.Kose O, Arca E, Akgul O, Erbil K. The levels of serum neopterin in Behcet's disease—objective marker of disease activity. J Dermatol Sci. 2006;42:128–30. doi: 10.1016/j.jdermsci.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Bank I, Duvdevani M, Livneh A. Expansion of gammadelta T-cells in Behcet's disease: role of disease activity and microbial flora in oral ulcers. J Lab Clin Med. 2003;141:33–40. doi: 10.1067/mlc.2003.1. [DOI] [PubMed] [Google Scholar]
- 91.Hamzaoui K, Hamzaoui A, Zakraoui L, Chabbou A. Expression of Bcl-2 in inflammatory sites from patients with active Behcet's disease. Mediators Inflamm. 1999;8:101–6. doi: 10.1080/09629359990595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Desbois AC, Wechsler B, Resche-Rigon M, Piette JC, Huong Dle T, Amoura Z, et al. Immunosuppressants reduce venous thrombosis relapse in Behcet's disease. Arthritis Rheum. 2012;64:2753–60. doi: 10.1002/art.34450. [DOI] [PubMed] [Google Scholar]
- 93.Geri G, Wechsler B, Thi Huong du L, Isnard R, Piette JC, Amoura Z, et al. Spectrum of cardiac lesions in Behcet disease: a series of 52 patients and review of the literature. Medicine. 2012;91:25–34. doi: 10.1097/MD.0b013e3182428f49. [DOI] [PubMed] [Google Scholar]
- 94.Saadoun D, Asli B, Wechsler B, Houman H, Geri G, Desseaux K, et al. Long-term outcome of arterial lesions in Behcet disease: a series of 101 patients. Medicine. 2012;91:18–24. doi: 10.1097/MD.0b013e3182428126. [DOI] [PubMed] [Google Scholar]
- 95.Ideguchi H, Suda A, Takeno M, Ueda A, Ohno S, Ishigatsubo Y. Characteristics of vascular involvement in Behcet's disease in Japan: a retrospective cohort study. Clin Exp Rheumatol. 2011;29(Suppl 67):S47–53. [PubMed] [Google Scholar]
- 96.Koksoy C, Gyedu A, Alacayir I, Bengisun U, Uncu H, Anadol E. Surgical treatment of peripheral aneurysms in patients with Behcet's disease. Eur J Vasc Endovasc Surg. 2011;42:525–30. doi: 10.1016/j.ejvs.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Riera-Mestre A, Martinez-Yelamos S, Martinez-Yelamos A, Ferrer I, Pujol R, Vidaller A. Clinicopathologic features and outcomes of neuro-Behcet disease in Spain: a study of 20 patients. Eur J Intern Med. 2010;21:536–41. doi: 10.1016/j.ejim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Hamuryudan V, Hatemi G, Tascilar K, Sut N, Ozyazgan Y, Seyahi E, et al. Prognosis of Behcet's syndrome among men with mucocutaneous involvement at disease onset: long-term outcome of patients enrolled in a controlled trial. Rheumatology. 2010;49:173–7. doi: 10.1093/rheumatology/kep350. [DOI] [PubMed] [Google Scholar]
- 99.Kural-Seyahi E, Fresko I, Seyahi N, Ozyazgan Y, Mat C, Hamuryudan V, et al. The long-term mortality and morbidity of Behcet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Medicine. 2003;82:60–76. doi: 10.1097/00005792-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 100.Tuzun H, Besirli K, Sayin A, Vural FS, Hamuryudan V, Hizli N, et al. Management of aneurysms in Behcet's syndrome: an analysis of 24 patients. Surgery. 1997;121:150–6. doi: 10.1016/s0039-6060(97)90284-1. [DOI] [PubMed] [Google Scholar]
- 101.Yazici H, Basaran G, Hamuryudan V, Hizli N, Yurdakul S, Mat C, et al. The ten-year mortality in Behcet's syndrome. Br J Rheumatol. 1996;35:139–41. doi: 10.1093/rheumatology/35.2.139. [DOI] [PubMed] [Google Scholar]
- 102.Hamuryudan V, Yurdakul S, Moral F, Numan F, Tuzun H, Tuzuner N, et al. Pulmonary arterial aneurysms in Behcet's syndrome: a report of 24 cases. Br J Rheumatol. 1994;33:48–51. doi: 10.1093/rheumatology/33.1.48. [DOI] [PubMed] [Google Scholar]
- 103.Saadoun D, Wechsler B, Desseaux K, Le Thi Huong D, Amoura Z, Resche-Rigon M, et al. Mortality in Behcet's disease. Arthritis Rheum. 2010;62:2806–12. doi: 10.1002/art.27568. [DOI] [PubMed] [Google Scholar]
- 104.Kim SK, Jang WC, Ahn YC, Lee SH, Lee SS, Hur JW. Promoter –2518 single nucleotide polymorphism of monocyte chemoattractant protein-1 is associated with clinical severity in Behcet's disease. Inflamm Res. 2012;61:541–5. doi: 10.1007/s00011-012-0471-5. [DOI] [PubMed] [Google Scholar]
- 105.Choe JY, Chung WT, Lee SW, Lee SS, Choi CB, Park SH, et al. Regional distinction for the clinical severity of Behcet's disease in Korea: four university-based medical centers study. Clin Exp Rheumatol. 2010;28(Suppl 60):S20–6. [PubMed] [Google Scholar]
- 106.Arabaci T, Kara C, Cicek Y. Relationship between periodontal parameters and Behcet's disease and evaluation of different treatments for oral recurrent aphthous stomatitis. J Periodontal Res. 2009;44:718–25. doi: 10.1111/j.1600-0765.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- 107.Aksu K, Kitapcioglu G, Keser G, Berdeli A, Karabulut G, Kobak S, et al. FcgammaRIIa, IIIa and IIIb gene polymorphisms in Behcet's disease: do they have any clinical implications? Clin Exp Rheumatol. 2008;26(Suppl 50):S77–83. [PubMed] [Google Scholar]
- 108.Alpsoy E, Donmez L, Onder M, Gunasti S, Usta A, Karincaoglu Y, et al. Clinical features and natural course of Behcet's disease in 661 cases: a multicentre study. Br J Dermatol. 2007;157:901–6. doi: 10.1111/j.1365-2133.2007.08116.x. [DOI] [PubMed] [Google Scholar]
- 109.Rozenbaum M, Boulman N, Slobodin G, Zisman D, Mader R, Yankevitch A, et al. Behcet disease in adult Druzes in north Israel: the influence of ethnic origin on disease expression and severity. J Clin Rheumatol. 2007;13:124–7. doi: 10.1097/RHU.0b013e3180645878. [DOI] [PubMed] [Google Scholar]
- 110.Akman A, Kacaroglu H, Donmez L, Bacanli A, Alpsoy E. Relationship between periodontal findings and Behcet's disease: a controlled study. J Clin Periodontol. 2007;34:485–91. doi: 10.1111/j.1600-051X.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 111.Inanc N, Mumcu G, Birtas E, Elbir Y, Yavuz S, Ergun T, et al. Serum mannose-binding lectin levels are decreased in Behcet's disease and associated with disease severity. J Rheumatol. 2005;32:287–91. [PubMed] [Google Scholar]
- 112.Mumcu G, Ergun T, Inanc N, Fresko I, Atalay T, Hayran O, et al. Oral health is impaired in Behcet's disease and is associated with disease severity. Rheumatology. 2004;43:1028–33. doi: 10.1093/rheumatology/keh236. [DOI] [PubMed] [Google Scholar]
- 113.Krause I, Mader R, Sulkes J, Paul M, Uziel Y, Adawi M, et al. Behcet's disease in Israel: the influence of ethnic origin on disease expression and severity. J Rheumatol. 2001;28:1033–6. [PubMed] [Google Scholar]
- 114.Krause I, Rosen Y, Kaplan I, Milo G, Guedj D, Molad Y, et al. Recurrent aphthous stomatitis in Behcet's disease: clinical features and correlation with systemic disease expression and severity. J Oral Pathol Med. 1999;28:193–6. doi: 10.1111/j.1600-0714.1999.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 115.Polat M, Vahaboglu G, Onde U, Eksioglu M. Classifying patients with Behcet's disease for disease severity, using a discriminating analysis method. Clin Exp Dermatol. 2009;34:151–5. doi: 10.1111/j.1365-2230.2008.02811.x. [DOI] [PubMed] [Google Scholar]
- 116.Park SH, Park KS, Seo YI, Min DJ, Kim WU, Kim TG, et al. Association of MICA polymorphism with HLA-B51 and disease severity in Korean patients with Behcet's disease. J Korean Med Sci. 2002;17:366–70. doi: 10.3346/jkms.2002.17.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gul A, Uyar FA, Inanc M, Ocal L, Tugal-Tutkun I, Aral O, et al. Lack of association of HLA-B*51 with a severe disease course in Behcet's disease. Rheumatology. 2001;40:668–72. doi: 10.1093/rheumatology/40.6.668. [DOI] [PubMed] [Google Scholar]
- 118.Fani MM, Ebrahimi H, Pourshahidi S, Aflaki E, Shafiee Sarvestani S. Comparing the effect of phenytoin syrup and triamcinolone acetonide ointment on aphthous ulcers in patients with Behcet's syndrome. Iran Red Crescent Med J. 2012;14:75–8. [PMC free article] [PubMed] [Google Scholar]
- 119.Qiao H, Sonoda KH, Ariyama A, Kuratomi Y, Kawano Y, Ishibashi T. CXCR2 Expression on neutrophils is upregulated during the relapsing phase of ocular Behcet disease. Curr Eye Res. 2005;30:195–203. doi: 10.1080/02713680490904331. [DOI] [PubMed] [Google Scholar]
- 120.Alpsoy E, Durusoy C, Yilmaz E, Ozgurel Y, Ermis O, Yazar S, et al. Interferon alfa-2a in the treatment of Behcet disease: a randomized placebo-controlled and double-blind study. Arch Dermatol. 2002;138:467–71. doi: 10.1001/archderm.138.4.467. [DOI] [PubMed] [Google Scholar]
- 121.Uzun S, Alpsoy E, Durdu M, Akman A. The clinical course of Behcet's disease in pregnancy: a retrospective analysis and review of the literature. J Dermatol. 2003;30:499–502. doi: 10.1111/j.1346-8138.2003.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 122.Hamuryudan V, Hatemi G, Sut N, Ugurlu S, Yurdakul S, Yazici H. Frequent oral ulceration during early disease may predict a severe disease course in males with Behçet's syndrome. Clin Exp Rheumatol. 2012;30(Suppl 72):32–4. [PubMed] [Google Scholar]
- 123.Arida A, Fragiadaki K, Giavri E, Sfikakis PP. Anti-TNF agents for Behcet's disease: analysis of published data on 369 patients. Semin Arthritis Rheum. 2011;41:61–70. doi: 10.1016/j.semarthrit.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 124.Gilworth G, Chamberlain MA, Bhakta B, Haskard D, Silman A, Tennant A. Development of the BD-QoL: a quality of life measure specific to Behcet's disease. J Rheumatol. 2004;31:931–7. [PubMed] [Google Scholar]
- 125.Touma Z, Ghandour L, Sibai A, Puzantian H, Hamdan A, Hamdan O, et al. Cross-cultural adaptation and validation of Behcet's disease quality of life questionnaire. BMC Med Res Methodol. 2011;11:52. doi: 10.1186/1471-2288-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mumcu G, Sur H, Inanc N, Karacayli U, Cimilli H, Sisman N, et al. A composite index for determining the impact of oral ulcer activity in Behcet's disease and recurrent aphthous stomatitis. J Oral Pathol Med. 2009;38:785–91. doi: 10.1111/j.1600-0714.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- 127.Karacayli U, Mumcu G, Simsek I, Pay S, Kose O, Erdem H, et al. The close association between dental and periodontal treatments and oral ulcer course in Behcet's disease: a prospective clinical study. J Oral Pathol Med. 2009;38:410–5. doi: 10.1111/j.1600-0714.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 128.Mumcu G, Niazi S, Stewart J, Hagi-Pavli E, Gokani B, Seoudi N, et al. Oral health and related quality of life status in patients from UK and Turkey: a comparative study in Behcet's disease. J Oral Pathol Med. 2009;38:406–9. doi: 10.1111/j.1600-0714.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- 129.Chams-Davatchi C, Barikbin B, Shahram F, Nadji A, Moghaddassi M, Yousefi M, et al. Pimecrolimus versus placebo in genital aphthous ulcers of Behcet's disease: a randomized double-blind controlled trial. Int J Rheum Dis. 2010;13:253–8. doi: 10.1111/j.1756-185X.2010.01531.x. [DOI] [PubMed] [Google Scholar]
- 130.Kose O, Dinc A, Simsek I. Randomized trial of pimecrolimus cream plus colchicine tablets versus colchicine tablets in the treatment of genital ulcers in Behcet's disease. Dermatology. 2009;218:140–5. doi: 10.1159/000182257. [DOI] [PubMed] [Google Scholar]
- 131.Mumcu G, Hayran O, Ozalp DO, Inanc N, Yavuz S, Ergun T, et al. The assessment of oral health-related quality of life by factor analysis in patients with Behcet's disease and recurrent aphthous stomatitis. J Oral Pathol Med. 2007;36:147–52. doi: 10.1111/j.1600-0714.2007.00514.x. [DOI] [PubMed] [Google Scholar]
- 132.Mat C, Yurdakul S, Uysal S, Gogus F, Ozyazgan Y, Uysal O, et al. A double-blind trial of depot corticosteroids in Behcet's syndrome. Rheumatology. 2006;45:348–52. doi: 10.1093/rheumatology/kei165. [DOI] [PubMed] [Google Scholar]
- 133.Melikoglu M, Fresko I, Mat C, Ozyazgan Y, Gogus F, Yurdakul S, et al. Short-term trial of etanercept in Behcet's disease: a double blind, placebo controlled study. J Rheumatol. 2005;32:98–105. [PubMed] [Google Scholar]
- 134.Matsuda T, Ohno S, Hirohata S, Miyanaga Y, Ujihara H, Inaba G, et al. Efficacy of rebamipide as adjunctive therapy in the treatment of recurrent oral aphthous ulcers in patients with Behcet's disease: a randomised, double-blind, placebo-controlled study. Drugs R D. 2003;4:19–28. doi: 10.2165/00126839-200304010-00002. [DOI] [PubMed] [Google Scholar]
- 135.Sharquie KE, Najim RA, Abu-Raghif AR. Dapsone in Behcet's disease: a double-blind, placebo-controlled, cross-over study. J Dermatol. 2002;29:267–79. doi: 10.1111/j.1346-8138.2002.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 136.Yurdakul S, Mat C, Tuzun Y, Ozyazgan Y, Hamuryudan V, Uysal O, et al. A double-blind trial of colchicine in Behcet's syndrome. Arthritis Rheum. 2001;44:2686–92. doi: 10.1002/1529-0131(200111)44:11<2686::aid-art448>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 137.Hamuryudan V, Mat C, Saip S, Ozyazgan Y, Siva A, Yurdakul S, et al. Thalidomide in the treatment of the mucocutaneous lesions of the Behcet syndrome. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:443–50. doi: 10.7326/0003-4819-128-6-199803150-00004. [DOI] [PubMed] [Google Scholar]
- 138.Calguneri M, Ertenli I, Kiraz S, Erman M, Celik I. Effect of prophylactic benzathine penicillin on mucocutaneous symptoms of Behcet's disease. Dermatology. 1996;192:125–8. doi: 10.1159/000246336. [DOI] [PubMed] [Google Scholar]
- 139.Hamuryudan V, Moral F, Yurdakul S, Mat C, Tuzun Y, Ozyazgan Y, et al. Systemic interferon alpha 2b treatment in Behcet's syndrome. J Rheumatol. 1994;21:1098–100. [PubMed] [Google Scholar]
- 140.Aktulga E, Altac M, Muftuoglu A, Ozyazgan Y, Pazarli H, Tuzun Y, et al. A double blind study of colchicine in Behcet's disease. Haematologica. 1980;65:399–402. [PubMed] [Google Scholar]
- 141.Gur A, Sarac AJ, Burkan YK, Nas K, Cevik R. Arthropathy, quality of life, depression, and anxiety in Behcet's disease: relationship between arthritis and these factors. Clin Rheumatol. 2006;25:524–31. doi: 10.1007/s10067-005-0100-6. [DOI] [PubMed] [Google Scholar]
- 142.Calguneri M, Kiraz S, Ertenli I, Benekli M, Karaarslan Y, Celik I. The effect of prophylactic penicillin treatment on the course of arthritis episodes in patients with Behcet's disease. A randomized clinical trial. Arthritis Rheum. 1996;39:2062–5. doi: 10.1002/art.1780391216. [DOI] [PubMed] [Google Scholar]
- 143.Moral F, Hamuryudan V, Yurdakul S, Yazici H. Inefficacy of azapropazone in the acute arthritis of Behcet's syndrome: a randomized, double blind, placebo controlled study. Clin Exp Rheumatol. 1995;13:493–5. [PubMed] [Google Scholar]
- 144.Hu K, Lei B, Kijlstra A, Li P, Zhang X, Xiao X, et al. Male sex, erythema nodosum, and electroretinography as predictors of visual prognosis after cataract surgery in patients with Behcet disease. J Cataract Refract Surg. 2012;38:1382–8. doi: 10.1016/j.jcrs.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 145.Okada AA, Goto H, Ohno S, Mochizuki M. Multicenter study of infliximab for refractory uveoretinitis in Behcet disease. Arch Ophthalmol. 2012;130:592–8. doi: 10.1001/archophthalmol.2011.2698. [DOI] [PubMed] [Google Scholar]
- 146.Diaz-Llopis M, Salom D, Garcia-de-Vicuna C, Cordero-Coma M, Ortega G, Ortego N, et al. Treatment of refractory uveitis with adalimumab: a prospective multicenter study of 131 patients. Ophthalmology. 2012;119:1575–81. doi: 10.1016/j.ophtha.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 147.Cantini F, Niccoli L, Nannini C, Kaloudi O, Cassara E, Susini M, et al. Efficacy of infliximab in refractory Behcet's disease-associated and idiopathic posterior segment uveitis: a prospective, follow-up study of 50 patients. Biologics. 2012;6:5–12. doi: 10.2147/BTT.S27343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Taylor SR, Singh J, Menezo V, Wakefield D, McCluskey P, Lightman S. Behcet disease: visual prognosis and factors influencing the development of visual loss. Am J Ophthalmol. 2011;152:1059–66. doi: 10.1016/j.ajo.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 149.Chu M, Yang P, Hou S, Li F, Chen Y, Kijlstra A. Behcet's disease exhibits an increased osteopontin serum level in active stage but no association with osteopontin and its receptor gene polymorphisms. Hum Immunol. 2011;72:525–9. doi: 10.1016/j.humimm.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 150.Onal S, Kazokoglu H, Koc A, Akman M, Bavbek T, Direskeneli H, et al. Long-term efficacy and safety of low-dose and dose-escalating interferon alfa-2a therapy in refractory Behcet uveitis. Arch Ophthalmol. 2011;129:288–94. doi: 10.1001/archophthalmol.2011.3. [DOI] [PubMed] [Google Scholar]
- 151.Onal S, Savar F, Akman M, Kazokoglu H. Vision- and health-related quality of life in patients with Behcet uveitis. Arch Ophthalmol. 2010;128:1265–71. doi: 10.1001/archophthalmol.2010.209. [DOI] [PubMed] [Google Scholar]
- 152.Davatchi F, Shahram F, Shams H, Nadji A, Chams-Davatchi C, Akhlaghi M, et al. Gender influence on ocular manifestations and their outcome in Behcet's Disease. A long-term follow-up of up to 20 years. Clin Rheumatol. 2011;30:541–7. doi: 10.1007/s10067-010-1574-4. [DOI] [PubMed] [Google Scholar]
- 153.Davatchi F, Shams H, Rezaipoor M, Sadeghi-Abdollahi B, Shahram F, Nadji A, et al. Rituximab in intractable ocular lesions of Behcet's disease; randomized single-blind control study (pilot study). Int J Rheum Dis. 2010;13:246–52. doi: 10.1111/j.1756-185X.2010.01546.x. [DOI] [PubMed] [Google Scholar]
- 154.Saadoun D, Wechsler B, Terrada C, Hajage D, Le Thi Huong D, Resche-Rigon M, et al. Azathioprine in severe uveitis of Behcet's disease. Arthritis Care Res. 2010;62:1733–8. doi: 10.1002/acr.20308. [DOI] [PubMed] [Google Scholar]
- 155.Sobaci G, Erdem U, Durukan AH, Erdurman C, Bayer A, Koksal S, et al. Safety and effectiveness of interferon alpha-2a in treatment of patients with Behcet's uveitis refractory to conventional treatments. Ophthalmology. 2010;117:1430–5. doi: 10.1016/j.ophtha.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 156.Yamada Y, Sugita S, Tanaka H, Kamoi K, Kawaguchi T, Mochizuki M. Comparison of infliximab versus ciclosporin during the initial 6-month treatment period in Behcet disease. Br J Ophthalmol. 2010;94:284–8. doi: 10.1136/bjo.2009.158840. [DOI] [PubMed] [Google Scholar]
- 157.Kramer M, Amer R, Mukamel M, Snir M, Jaouni T, Friling R. Uveitis in juvenile Behcet's disease: clinical course and visual outcome compared with adult patients. Eye (Lond) 2009;23:2034–41. doi: 10.1038/eye.2008.397. [DOI] [PubMed] [Google Scholar]
- 158.Gueudry J, Wechsler B, Terrada C, Gendron G, Cassoux N, Fardeau C, et al. Long-term efficacy and safety of low-dose interferon alpha2a therapy in severe uveitis associated with Behcet disease. Am J Ophthalmol. 2008;146:837–44. e1. doi: 10.1016/j.ajo.2008.08.038. [DOI] [PubMed] [Google Scholar]
- 159.Kump LI, Moeller KL, Reed GF, Kurup SK, Nussenblatt RB, Levy-Clarke GA. Behcet's disease: comparing 3 decades of treatment response at the National Eye Institute. Can J Ophthalmol. 2008;43:468–72. doi: 10.1139/i08-080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cho YJ, Kim WK, Lee JH, Byeon SH, Koh HJ, Kwon OW, et al. Visual prognosis and risk factors for Korean patients with Behcet uveitis. Ophthalmologica. 2008;222:344–50. doi: 10.1159/000146080. [DOI] [PubMed] [Google Scholar]
- 161.Krause L, Altenburg A, Pleyer U, Kohler AK, Zouboulis CC, Foerster MH. Longterm visual prognosis of patients with ocular Adamantiades-Behcet's disease treated with interferon-alpha-2a. J Rheumatol. 2008;35:896–903. [PubMed] [Google Scholar]
- 162.Yalcindag FN, Can E, Ozdemir O. Intravenous methylprednisolone pulse therapy for acute posterior segment uveitis attacks in Behcet's disease. Ann Ophthalmol (Skokie) 2007;39:194–7. doi: 10.1007/s12009-007-0018-5. [DOI] [PubMed] [Google Scholar]
- 163.Salvarani C, Pipitone N, Catanoso MG, Cimino L, Tumiati B, Macchioni P, et al. Epidemiology and clinical course of Behcet's disease in the Reggio Emilia area of Northern Italy: a seventeen-year population-based study. Arthritis Rheum. 2007;57:171–8. doi: 10.1002/art.22500. [DOI] [PubMed] [Google Scholar]
- 164.Tugal-Tutkun I, Guney-Tefekli E, Urgancioglu M. Results of interferon-alfa therapy in patients with Behcet uveitis. Graefes Arch Clin Exp Ophthalmol. 2006;244:1692–5. doi: 10.1007/s00417-006-0346-y. [DOI] [PubMed] [Google Scholar]
- 165.Tugal-Tutkun I, Mudun A, Urgancioglu M, Kamali S, Kasapoglu E, Inanc M, et al. Efficacy of infliximab in the treatment of uveitis that is resistant to treatment with the combination of azathioprine, cyclosporine, and corticosteroids in Behcet's disease: an open-label trial. Arthritis Rheum. 2005;52:2478–84. doi: 10.1002/art.21231. [DOI] [PubMed] [Google Scholar]
- 166.Takeuchi M, Hokama H, Tsukahara R, Kezuka T, Goto H, Sakai J, et al. Risk and prognostic factors of poor visual outcome in Behcet's disease with ocular involvement. Graefes Arch Clin Exp Ophthalmol. 2005;243:1147–52. doi: 10.1007/s00417-005-0005-8. [DOI] [PubMed] [Google Scholar]
- 167.Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, Huseyin Altunbas H, Urgancioglu M. Uveitis in Behcet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138:373–80. doi: 10.1016/j.ajo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 168.Lashay AR, Rahimi A, Chams H, Davatchi F, Shahram F, Hatmi ZN, et al. Evaluation of the effect of acetazolamide on cystoid macular oedema in patients with Behcet's disease. Eye (Lond) 2003;17:762–6. doi: 10.1038/sj.eye.6700464. [DOI] [PubMed] [Google Scholar]
- 169.Calguneri M, Ozturk MA, Ertenli I, Kiraz S, Apras S, Ozbalkan Z. Effects of interferon alpha treatment on the clinical course of refractory Behcet's disease: an open study. Ann Rheum Dis. 2003;62:492–3. doi: 10.1136/ard.62.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ozdal PC, Ortac S, Taskintuna I, Firat E. Long-term therapy with low dose cyclosporin A in ocular Behcet's disease. Doc Ophthalmol. 2002;105:301–12. doi: 10.1023/a:1021227019915. [DOI] [PubMed] [Google Scholar]
- 171.Ozerturk Y, Bardak Y, Durmus M. Vitreoretinal surgery in Behcet's disease with severe ocular complications. Acta Ophthalmol Scand. 2001;79:192–6. doi: 10.1034/j.1600-0420.2001.079002192.x. [DOI] [PubMed] [Google Scholar]
- 172.Ando K, Fujino Y, Hijikata K, Izawa Y, Masuda K. Epidemiological features and visual prognosis of Behcet's disease. Jpn J Ophthalmol. 1999;43:312–7. doi: 10.1016/s0021-5155(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 173.Fujino Y, Joko S, Masuda K, Yagi I, Kogure M, Sakai J, et al. Ciclosporin microemulsion preconcentrate treatment of patients with Behcet's disease. Jpn J Ophthalmol. 1999;43:318–26. doi: 10.1016/s0021-5155(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 174.Hamuryudan V, Ozyazgan Y, Hizli N, Mat C, Yurdakul S, Tuzun Y, et al. Azathioprine in Behcet's syndrome: effects on long-term prognosis. Arthritis Rheum. 1997;40:769–74. doi: 10.1002/art.1780400425. [DOI] [PubMed] [Google Scholar]
- 175.Sakamoto M, Akazawa K, Nishioka Y, Sanui H, Inomata H, Nose Y. Prognostic factors of vision in patients with Behcet disease. Ophthalmology. 1995;102:317–21. doi: 10.1016/s0161-6420(95)31022-6. [DOI] [PubMed] [Google Scholar]
- 176.Palmares J, Castro-Correia J, Coutinho MF, Araujo D, Delgado L. Immunosuppression in Behcet's disease Clinical management and long-term visual outcome. Ocul Immunol Inflamm. 1995;3:99–106. doi: 10.3109/09273949509085037. [DOI] [PubMed] [Google Scholar]
- 177.Ouazzani B, Benchekroun N, el Aouni A, Hajji Z, Chaoui Z, Berraho-Hamani A. Résultat de la maladie de Behçet dans la pratique ophtalmologique au Maroc [French]. Outcome of Behcet disease in ophthalmologic practice in Morocco. J Fr Ophtalmol. 1995;18:373–5. [PubMed] [Google Scholar]
- 178.Hayasaka S, Kawamoto K, Noda S, Kodama T. Visual prognosis in patients with Behcet's disease receiving colchicine, systemic corticosteroid or cyclosporin. Ophthalmologica. 1994;208:210–3. doi: 10.1159/000310490. [DOI] [PubMed] [Google Scholar]
- 179.Sajjadi H, Soheilian M, Ahmadieh H, Hassanein K, Parvin M, Azarmina M, et al. Low dose cyclosporin-A therapy in Behcet's disease. J Ocul Pharmacol. 1994;10:553–60. doi: 10.1089/jop.1994.10.553. [DOI] [PubMed] [Google Scholar]
- 180.Towler HM, Lightman S. Visual prognosis in Behcet's disease. Ocul Immunol Inflamm. 1993;1:249–54. doi: 10.3109/09273949309085025. [DOI] [PubMed] [Google Scholar]
- 181.Ozyazgan Y, Yurdakul S, Yazici H, Tuzun B, Iscimen A, Tuzun Y, et al. Low dose cyclosporin A versus pulsed cyclophosphamide in Behcet's syndrome: a single masked trial. Br J Ophthalmol. 1992;76:241–3. doi: 10.1136/bjo.76.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cochereau-Massin I, Wechsler B, Le Hoang P, Le Thi Huong D, Girard B, Rousselie F, et al. Pronostic oculaire dans la maladie de Behçet [French]. Ocular prognosis in Behcet's disease. J Fr Ophtalmol. 1992;15:343–7. [PubMed] [Google Scholar]
- 183.Mochizuki M, Masuda K, Sakane T, Inaba G, Ito K, Kogure M, et al. A multicenter clinical open trial of FK 506 in refractory uveitis, including Behcet's disease. Japanese FK 506 Study Group on Refractory Uveitis. Transplant Proc. 1991;23:3343–6. [PubMed] [Google Scholar]
- 184.Kazokoglu H, Saatci O, Cuhadaroglu H, Eldem B. Long-term effects of cyclophosphamide and colchicine treatment in Behcet's disease. Ann Ophthalmol. 1991;23:148–51. [PubMed] [Google Scholar]
- 185.Yazici H, Pazarli H, Barnes CG, Tuzun Y, Ozyazgan Y, Silman A, et al. A controlled trial of azathioprine in Behcet's syndrome. N Engl J Med. 1990;322:281–5. doi: 10.1056/NEJM199002013220501. [DOI] [PubMed] [Google Scholar]
- 186.Benezra D, Cohen E. Treatment and visual prognosis in Behcet's disease. Br J Ophthalmol. 1986;70:589–92. doi: 10.1136/bjo.70.8.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pivetti Pezzi P, Gasparri V, De Liso P, Catarinelli G. Prognosis in Behcet's disease. Ann Ophthalmol. 1985;17:20–5. [PubMed] [Google Scholar]
- 188.Nussenblatt RB, Palestine AG, Chan CC. Cyclosporine therapy for uveitis: long-term followup. J Ocul Pharmacol. 1985;1:369–82. doi: 10.1089/jop.1985.1.369. [DOI] [PubMed] [Google Scholar]
- 189.Masuda K, Nakajima A, Urayama A, Nakae K, Kogure M, Inaba G. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behcet's disease. Lancet. 1989;1:1093–6. doi: 10.1016/s0140-6736(89)92381-7. [DOI] [PubMed] [Google Scholar]
- 190.BenEzra D, Cohen E, Chajek T, Friedman G, Pizanti S, de Courten C, et al. Evaluation of conventional therapy versus cyclosporine A in Behcet's syndrome. Transplant Proc. 1988;20(Suppl 4):136–43. [PubMed] [Google Scholar]
- 191.Simonini G, Taddio A, Cattalini M, Caputo R, De Libero C, Naviglio S, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res. 2011;63:612–8. doi: 10.1002/acr.20404. [DOI] [PubMed] [Google Scholar]
- 192.Sugita S, Yamada Y, Mochizuki M. Relationship between serum infliximab levels and acute uveitis attacks in patients with Behcet disease. Br J Ophthalmol. 2011;95:549–52. doi: 10.1136/bjo.2009.174888. [DOI] [PubMed] [Google Scholar]
- 193.Usui Y, Takeuchi M, Yamakawa N, Takeuchi A, Kezuka T, Ma J, et al. Expression and function of inducible costimulator on peripheral blood CD4+ T cells in Behcet's patients with uveitis: a new activity marker? Invest Ophthalmol Vis Sci. 2010;51:5099–104. doi: 10.1167/iovs.10-5286. [DOI] [PubMed] [Google Scholar]
- 194.Yamada Y, Sugita S, Tanaka H, Kamoi K, Takase H, Mochizuki M. Timing of recurrent uveitis in patients with Behcet's disease receiving infliximab treatment. Br J Ophthalmol. 2011;95:205–8. doi: 10.1136/bjo.2009.168856. [DOI] [PubMed] [Google Scholar]
- 195.Mudun BA, Ergen A, Ipcioglu SU, Burumcek EY, Durlu Y, Arslan MO. Short-term chlorambucil for refractory uveitis in Behcet's disease. Ocul Immunol Inflamm. 2001;9:219–29. doi: 10.1076/ocii.9.4.219.3957. [DOI] [PubMed] [Google Scholar]
- 196.Sakane T, Mochizuki M, Inaba G, Masuda K. A phase II study of FK506 (tacrolimus) on refractory uveitis associated with Behcet's disease and allied conditions [Japanese]. Ryumachi. 1995;35:802–13. [PubMed] [Google Scholar]
- 197.Ahn JK, Lee YS, Jeon CH, Koh EM, Cha HS. Treatment of venous thrombosis associated with Behcet's disease: immunosuppressive therapy alone versus immunosuppressive therapy plus anticoagulation. Clin Rheumatol. 2008;27:201–5. doi: 10.1007/s10067-007-0685-z. [DOI] [PubMed] [Google Scholar]
- 198.Cho SB, Kim T, Cho S, Shim WH, Yang MS, Bang D. Major arterial aneurysms and pseudoaneurysms in Behcet's disease: results from a single centre. Scand J Rheumatol. 2011;40:64–7. doi: 10.3109/03009742.2010.497161. [DOI] [PubMed] [Google Scholar]
- 199.Kim WH, Choi D, Kim JS, Ko YG, Jang Y, Shim WH. Effectiveness and safety of endovascular aneurysm treatment in patients with vasculo-Behcet disease. J Endovasc Ther. 2009;16:631–6. doi: 10.1583/09-2812.1. [DOI] [PubMed] [Google Scholar]
- 200.Park MC, Hong BK, Kwon HM, Hong YS. Surgical outcomes and risk factors for postoperative complications in patients with Behcet's disease. Clin Rheumatol. 2007;26:1475–80. doi: 10.1007/s10067-006-0530-9. [DOI] [PubMed] [Google Scholar]
- 201.Tuzun H, Seyahi E, Arslan C, Hamuryudan V, Besirli K, Yazici H. Management and prognosis of nonpulmonary large arterial disease in patients with Behcet disease. J Vasc Surg. 2012;55:157–63. doi: 10.1016/j.jvs.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 202.Zhu Y, Wu Q, Guo L, Fang L, Yan X, Zhang F, et al. The clinical characteristics and outcome of intracardiac thrombus and aortic valvular involvement in Behçet's disease: an analysis of 20 cases. Clin Exp Rheumatol. 2012;30(Suppl 72):40–5. [PubMed] [Google Scholar]
- 203.Ha Y, Jung S, Lee K, Jung S, Lee S, Park M, et al. Long-term clinical outcomes and risk factors for the occurrence of post-operative complications after cardiovascular surgery in patients with Behcet's disease. Clin Exp Rheumatol. 2012;30(Suppl 72):18–26. [PubMed] [Google Scholar]
- 204.Ait Ben Haddou EH, Imounan F, Regragui W, Mouti O, Benchakroune N, Abouqal R, et al. Neurological manifestations of Behcet's disease: evaluation of 40 patients treated by cyclophosphamide. Rev Neurol (Paris) 2012;168:344–9. doi: 10.1016/j.neurol.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 205.Akman-Demir G, Serdaroglu P, Tasci B. Clinical patterns of neurological involvement in Behcet's disease: evaluation of 200 patients. The Neuro-Behcet Study Group. Brain. 1999;122(Pt 11):2171–82. doi: 10.1093/brain/122.11.2171. [DOI] [PubMed] [Google Scholar]
- 206.Akman-Demir G, Tuzun E, Icoz S, Yesilot N, Yentur SP, Kurtuncu M, et al. Interleukin-6 in neuro-Behcet's disease: association with disease subsets and long-term outcome. Cytokine. 2008;44:373–6. doi: 10.1016/j.cyto.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 207.Evcik D, Dogan SK, Ay S, Cuzdan N, Guven M, Gurler A, et al. Does Behcet's disease associate with neuropathic pain syndrome and impaired well-being? Clin Rheumatol. 2013;32:33–6. doi: 10.1007/s10067-012-2086-1. [DOI] [PubMed] [Google Scholar]
- 208.Gunduz T, Emir O, Kurtuncu M, Mutlu M, Tumac A, Akca S, et al. Cognitive impairment in neuro-Behcet's disease and multiple sclerosis: a comparative study. Int J Neurosci. 2012;122:650–6. doi: 10.3109/00207454.2012.704454. [DOI] [PubMed] [Google Scholar]
- 209.Siva A, Kantarci OH, Saip S, Altintas A, Hamuryudan V, Islak C, et al. Behcet's disease: diagnostic and prognostic aspects of neurological involvement. J Neurol. 2001;248:95–103. doi: 10.1007/s004150170242. [DOI] [PubMed] [Google Scholar]
- 210.Yesilot N, Mutlu M, Gungor O, Baykal B, Serdaroglu P, Akman-Demir G. Clinical characteristics and course of spinal cord involvement in Behcet's disease. Eur J Neurol. 2007;14:729–37. doi: 10.1111/j.1468-1331.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 211.Cheon JH, Han DS, Park JY, Ye BD, Jung SA, Park YS, et al. Development, validation, and responsiveness of a novel disease activity index for intestinal Behcet's disease. Inflamm Bowel Dis. 2011;17:605–13. doi: 10.1002/ibd.21313. [DOI] [PubMed] [Google Scholar]
- 212.Choi CH, Kim TI, Kim BC, Shin SJ, Lee SK, Kim WH, et al. Anti-Saccharomyces cerevisiae antibody in intestinal Behcet's disease patients: relation to clinical course. Dis Colon Rectum. 2006;49:1849–59. doi: 10.1007/s10350-006-0706-z. [DOI] [PubMed] [Google Scholar]
- 213.Choi IJ, Kim JS, Cha SD, Jung HC, Park JG, Song IS, et al. Long-term clinical course and prognostic factors in intestinal Behcet's disease. Dis Colon Rectum. 2000;43:692–700. doi: 10.1007/BF02235590. [DOI] [PubMed] [Google Scholar]
- 214.Chung MJ, Cheon JH, Kim SU, Park JJ, Kim TI, Kim NK, et al. Response rates to medical treatments and long-term clinical outcomes of nonsurgical patients with intestinal Behcet disease. J Clin Gastroenterol. 2010;44:e116–22. doi: 10.1097/MCG.0b013e3181c8a50f. [DOI] [PubMed] [Google Scholar]
- 215.Jung YS, Cheon JH, Hong SP, Kim TI, Kim WH. Clinical outcomes and prognostic factors for thiopurine maintenance therapy in patients with intestinal Behcet's disease. Inflamm Bowel Dis. 2012;18:750–7. doi: 10.1002/ibd.21757. [DOI] [PubMed] [Google Scholar]
- 216.Jung YS, Cheon JH, Park SJ, Hong SP, Kim TI, Kim WH. Clinical course of intestinal Behcet's disease during the first five years. Dig Dis Sci. 2013;58:496–503. doi: 10.1007/s10620-012-2351-9. [DOI] [PubMed] [Google Scholar]
- 217.Jung YS, Cheon JH, Park SJ, Hong SP, Kim TI, Kim WH. Long-term clinical outcomes of Crohn's disease and intestinal Behcet's disease. Inflamm Bowel Dis. 2013;19:99–105. doi: 10.1002/ibd.22991. [DOI] [PubMed] [Google Scholar]
- 218.Jung YS, Hong SP, Kim TI, Kim WH, Cheon JH. Long-term clinical outcomes and factors predictive of relapse after 5-aminosalicylate or sulfasalazine therapy in patients with intestinal Behcet disease. J Clin Gastroenterol. 2012;46:e38–45. doi: 10.1097/MCG.0b013e3182431d56. [DOI] [PubMed] [Google Scholar]
- 219.Jung YS, Hong SP, Kim TI, Kim WH, Cheon JH. Early versus late surgery in patients with intestinal Behcet disease. Dis Colon Rectum. 2012;55:65–71. doi: 10.1097/DCR.0b013e318238b57e. [DOI] [PubMed] [Google Scholar]
- 220.Jung YS, Kim SW, Yoon JY, Lee JH, Jeon SM, Hong SP, et al. Expression of a soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) correlates with clinical disease activity in intestinal Behcet's disease. Inflamm Bowel Dis. 2011;17:2130–7. doi: 10.1002/ibd.21600. [DOI] [PubMed] [Google Scholar]
- 221.Jung YS, Yoon JY, Hong SP, Kim TI, Kim WH, Cheon JH. Influence of age at diagnosis and sex on clinical course and long-term prognosis of intestinal Behcet's disease. Inflamm Bowel Dis. 2012;18:1064–71. doi: 10.1002/ibd.21833. [DOI] [PubMed] [Google Scholar]
- 222.Jung YS, Yoon JY, Lee JH, Jeon SM, Hong SP, Kim TI, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behcet's disease. Inflamm Bowel Dis. 2011;17:1594–602. doi: 10.1002/ibd.21517. [DOI] [PubMed] [Google Scholar]
- 223.Kim JS, Lim SH, Choi IJ, Moon H, Jung HC, Song IS, et al. Prediction of the clinical course of Behcet's colitis according to macroscopic classification by colonoscopy. Endoscopy. 2000;32:635–40. doi: 10.1055/s-2000-9012. [DOI] [PubMed] [Google Scholar]
- 224.Kim WH, Cho YS, Yoo HM, Park IS, Park EC, Lim JG. Quality of life in Korean patients with inflammatory bowel diseases: ulcerative colitis, Crohn's disease and intestinal Behcet's disease. Int J Colorectal Dis. 1999;14:52–7. doi: 10.1007/s003840050183. [DOI] [PubMed] [Google Scholar]
- 225.Lee KS, Kim SJ, Lee BC, Yoon DS, Lee WJ, Chi HS. Surgical treatment of intestinal Behcet's disease. Yonsei Med J. 1997;38:455–60. doi: 10.3349/ymj.1997.38.6.455. [DOI] [PubMed] [Google Scholar]
- 226.Naganuma M, Iwao Y, Inoue N, Hisamatsu T, Imaeda H, Ishii H, et al. Analysis of clinical course and long-term prognosis of surgical and nonsurgical patients with intestinal Behcet's disease. Am J Gastroenterol. 2000;95:2848–51. doi: 10.1111/j.1572-0241.2000.03198.x. [DOI] [PubMed] [Google Scholar]
- 227.Shin SJ, Kim BC, Kim TI, Lee SK, Lee KH, Kim WH. Anti-alpha-enolase antibody as a serologic marker and its correlation with disease severity in intestinal Behcet's disease. Dig Dis Sci. 2011;56:812–8. doi: 10.1007/s10620-010-1326-y. [DOI] [PubMed] [Google Scholar]
- 228.Gul IG, Kartalci S, Cumurcu BE, Karincaoglu Y, Yologlu S, Karlidag R. Evaluation of sexual function in patients presenting with Behcet's disease with or without depression. J Eur Acad Dermatol Venereol. 2013;27:1244–51. doi: 10.1111/j.1468-3083.2012.04698.x. [DOI] [PubMed] [Google Scholar]
- 229.Bernabe E, Marcenes W, Mather J, Phillips C, Fortune F. Impact of Behcet's syndrome on health-related quality of life: influence of the type and number of symptoms. Rheumatology. 2010;49:2165–71. doi: 10.1093/rheumatology/keq251. [DOI] [PubMed] [Google Scholar]
- 230.Calikoglu E, Onder M, Cosar B, Candansayar S. Depression, anxiety levels and general psychological profile in Behcet's disease. Dermatology. 2001;203:238–40. doi: 10.1159/000051756. [DOI] [PubMed] [Google Scholar]
- 231.Cavaco S, da Silva AM, Pinto P, Coutinho E, Santos E, Bettencourt A, et al. Cognitive functioning in Behcet's disease. Ann N Y Acad Sci. 2009;1173:217–26. doi: 10.1111/j.1749-6632.2009.04670.x. [DOI] [PubMed] [Google Scholar]
- 232.Erberk-Ozen N, Birol A, Boratav C, Kocak M. Executive dysfunctions and depression in Behcet's disease without explicit neurological involvement. Psychiatry Clin Neurosci. 2006;60:465–72. doi: 10.1111/j.1440-1819.2006.01533.x. [DOI] [PubMed] [Google Scholar]
- 233.Gurbuzler L, Inanir A, Yelken K, Koc S, Eyibilen A, Uysal IO, et al. Behcet's disease impairs voice quality without laryngeal and hypopharyngeal involvement. Eur Arch Otorhinolaryngol. 2012;269:2539–42. doi: 10.1007/s00405-012-2100-x. [DOI] [PubMed] [Google Scholar]
- 234.Hayran O, Mumcu G, Inanc N, Ergun T, Direskeneli H. Assessment of minimal clinically important improvement by using Oral Health Impact Profile-14 in Behcet's disease. Clin Exp Rheumatol. 2009;27(Suppl 53):S79–84. [PubMed] [Google Scholar]
- 235.Hiz O, Ediz L, Gulcu E, Tekeoglu I. Effects of Behcet's disease on sexual function and psychological status of male patients. J Sex Med. 2011;8:1426–33. doi: 10.1111/j.1743-6109.2010.02040.x. [DOI] [PubMed] [Google Scholar]
- 236.Kocak M, Basar MM, Vahapoglu G, Mert HC, Gungor S. The effect of Behcet's disease on sexual function and psychiatric status of premenopausal women. J Sex Med. 2009;6:1341–8. doi: 10.1111/j.1743-6109.2008.01078.x. [DOI] [PubMed] [Google Scholar]
- 237.Pincus T, Sokka T. Can a Multi-Dimensional Health Assessment Questionnaire (MDHAQ) and Routine Assessment of Patient Index Data (RAPID) scores be informative in patients with all rheumatic diseases? Best Pract Res Clin Rheumatol. 2007;21:733–53. doi: 10.1016/j.berh.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 238.Taner E, Cosar B, Burhanoglu S, Calikoglu E, Onder M, Arikan Z. Depression and anxiety in patients with Behcet's disease compared with that in patients with psoriasis. Int J Dermatol. 2007;46:1118–24. doi: 10.1111/j.1365-4632.2007.03247.x. [DOI] [PubMed] [Google Scholar]
- 239.Tanriverdi N, Taskintuna, Duru C, Ozdal P, Ortac S, Firat E. Health-related quality of life in Behcet patients with ocular involvement. Jpn J Ophthalmol. 2003;47:85–92. doi: 10.1016/s0021-5155(02)00647-0. [DOI] [PubMed] [Google Scholar]
- 240.Uguz F, Dursun R, Kaya N, Cilli AS. Quality of life in patients with Behcet's disease: the impact of major depression. Gen Hosp Psychiatry. 2007;29:21–4. doi: 10.1016/j.genhosppsych.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 241.Karlidag R, Unal S, Evereklioglu C, Sipahi B, Er H, Yologlu S. Stressful life events, anxiety, depression and coping mechanisms in patients with Behcet's disease. J Eur Acad Dermatol Venereol. 2003;17:670–5. doi: 10.1046/j.1468-3083.2003.00760.x. [DOI] [PubMed] [Google Scholar]
- 242.Sut N, Seyahi E, Yurdakul S, Senocak M, Yazici H. A cost analysis of Behcet's syndrome in Turkey. Rheumatology. 2007;46:678–82. doi: 10.1093/rheumatology/kel382. [DOI] [PubMed] [Google Scholar]
- 243.Alpsoy E, Er H, Durusoy C, Yilmaz E. The use of sucralfate suspension in the treatment of oral and genital ulceration of Behcet disease: a randomized, placebo-controlled, double-blind study. Arch Dermatol. 1999;135:529–32. doi: 10.1001/archderm.135.5.529. [DOI] [PubMed] [Google Scholar]
- 244.Avci O, Gurler N, Gunes AT. Efficacy of cyclosporine on mucocutaneous manifestations of Behcet's disease. J Am Acad Dermatol. 1997;36(Pt 1):796–7. doi: 10.1016/s0190-9622(97)80354-4. [DOI] [PubMed] [Google Scholar]
- 245.Kaneko F, Oyama N, Nishibu A. Streptococcal infection in the pathogenesis of Behcet's disease and clinical effects of minocycline on the disease symptoms. Yonsei Med J. 1997;38:444–54. doi: 10.3349/ymj.1997.38.6.444. [DOI] [PubMed] [Google Scholar]
- 246.Davies UM, Palmer RG, Denman AM. Treatment with acyclovir does not affect orogenital ulcers in Behcet's syndrome: a randomized double-blind trial. Br J Rheumatol. 1988;27:300–2. doi: 10.1093/rheumatology/27.4.300. [DOI] [PubMed] [Google Scholar]
- 247.Hamuryudan V, Yurdakul S, Rosenkaimer F, Yazici H. Inefficacy of topical alpha interferon in the treatment of oral ulcers of Behcet's syndrome: a randomized, double blind trial. Br J Rheumatol. 1991;30:395–6. doi: 10.1093/rheumatology/30.5.395-a. [DOI] [PubMed] [Google Scholar]
- 248.Tasli L, Mat C, De Simone C, Yazici H. Lactobacilli lozenges in the management of oral ulcers of Behcet's syndrome. Clin Exp Rheumatol. 2006;5(Suppl 42):S83–6. [PubMed] [Google Scholar]
- 249.Ergun T, Gurbuz O, Yurdakul S, Hamuryudan V, Bekiroglu N, Yazici H. Topical cyclosporine-A for treatment of oral ulcers of Behcet's syndrome. Int J Dermatol. 1997;36:720. doi: 10.1111/j.1365-4362.1997.tb03133.x. [DOI] [PubMed] [Google Scholar]
- 250.Hamza MH. Treatment of Behcet's disease with thalidomide. Clin Rheumatol. 1986;5:365–71. doi: 10.1007/BF02054255. [DOI] [PubMed] [Google Scholar]
- 251.Boyvat A, Sisman-Solak C, Gurler A. Long-term effects of interferon alpha 2A treatment in Behcet's disease. Dermatology. 2000;201:40–3. doi: 10.1159/000018427. [DOI] [PubMed] [Google Scholar]
- 252.Martenet AC, Paccolat F. Traitement immunosuppresseur du syndrome de Behçet. Les résultats à long terme [French]. Immunosuppressive treatment of Behcet's syndrome. Long-term results. Ophtalmologie. 1989;3:40–2. [PubMed] [Google Scholar]
- 253.Zaghetto JM, Yamamoto MM, Souza MB, Silva FT, Hirata CE, Olivalves E, et al. Chlorambucil and cyclosporine A in Brazilian patients with Behcet's disease uveitis: a retrospective study. Arq Bras Oftalmol. 2010;73:40–6. doi: 10.1590/s0004-27492010000100007. [DOI] [PubMed] [Google Scholar]
- 254.Kim WU, Chung SM, Han TW, Sah WJ, Kim MH. Elevated soluble Fas in aqueous humor of patients with Behcet's uveitis: correlation with uveitis severity. Jpn J Ophthalmol. 2002;46:18–23. doi: 10.1016/s0021-5155(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 255.Blackford S, Finlay AY, Roberts DL. Quality of life in Behcet's syndrome: 335 patients surveyed. Br J Dermatol. 1997;136:293. doi: 10.1111/j.1365-2133.1997.tb14924.x. [DOI] [PubMed] [Google Scholar]
- 256.Moses Alder N, Fisher M, Yazici Y. Behcet's syndrome patients have high levels of functional disability, fatigue and pain as measured by a Multi-dimensional Health Assessment Questionnaire (MDHAQ). Clin Exp Rheumatol. 2008;26(Suppl 50):S110–3. [PubMed] [Google Scholar]