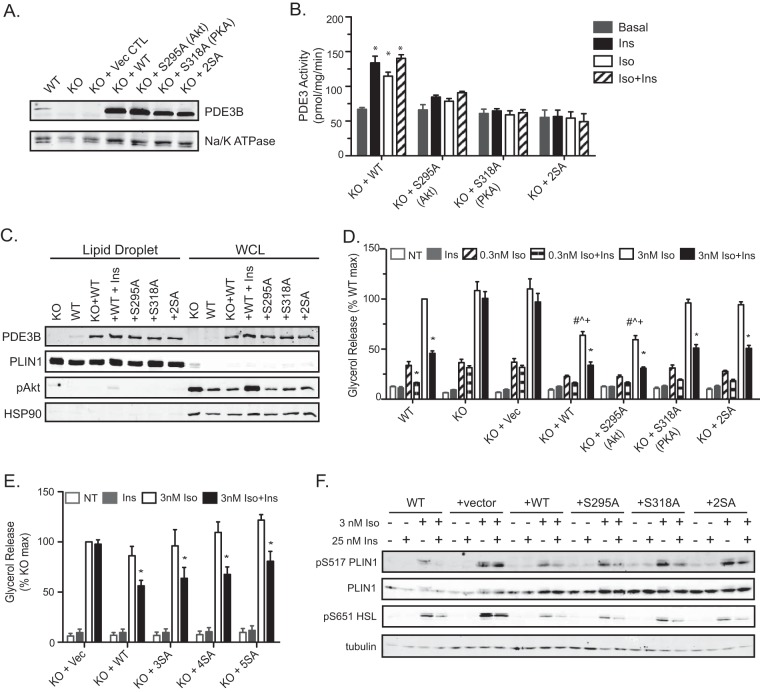
FIG 4.
Phosphorylation of PDE3B at putative Akt and PKA sites is not required for the antilipolytic effect of insulin in brown adipocytes. (A) Immunoblot for PDE3B protein in crude membrane lysates harvested from WT and Pde3b-KO brown adipocytes and from KO adipocytes overexpressing the indicated human PDE3B proteins. Na/K ATPase was used as a membrane loading control. (B) PDE3 activity in membrane fractions. Values represent results for 2 to 3 independent experiments. *, P < 0.001. (C) Representative immunoblot of the lipid droplet fraction and whole-cell lysate (WCL) for PDE3B using perilipin 1 and HSP90 as lipid droplet and cytosolic markers, respectively. For “+WT+Ins” samples, KO plus WT adipocytes were treated with 100 nM insulin for 15 minutes prior to fractionation. (D and E) Glycerol release was measured without Iso or upon the addition of 0.3 or 3 nM Iso (D) or 3 nM Iso (E) in the presence or absence of 25 nM Ins. Data are expressed as percentages of the response of WT (D) or Pde3b-KO (E) adipocytes upon stimulation with 3 nM Iso. Vec, vector; NT, no treatment (basal). Values represent results for 3 experiments. Symbols indicate significant differences (P < 0.05) for insulin suppression (*), for comparison with the WT group with 3 nM Iso (#), for comparison with the KO-plus-S318A group with 3 nM Iso (^), or for comparison with the KO-plus-2SA group with 3 nM Iso (+). (F) Representative immunoblots for phosphorylated perilipin 1 (PLIN1) and HSL in whole-cell lysates harvested at the end of the glycerol release assays. All data are presented as means ± standard errors of the means. Statistical analysis was performed using one-way ANOVA and Tukey's posttest.