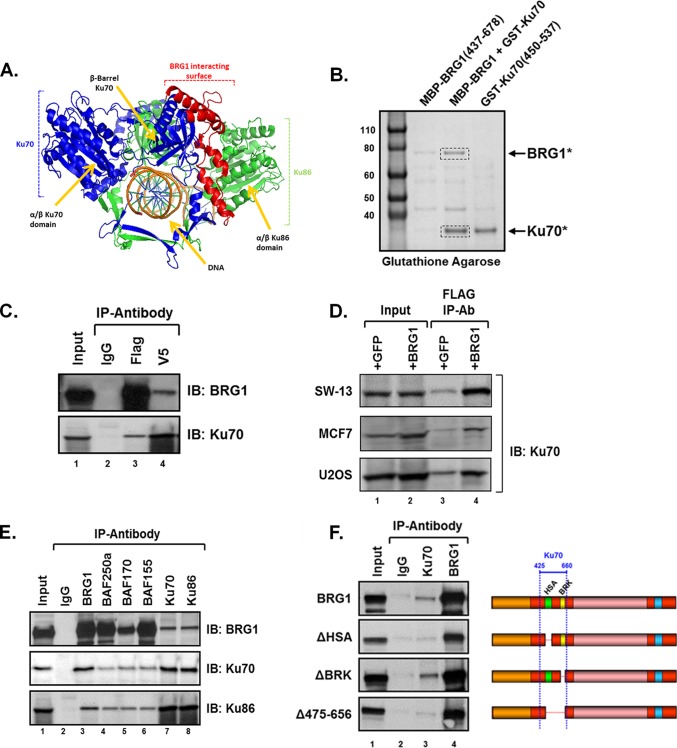
FIG 1.
Ku associates with the BRG1-SWI/SNF complex. Protein pulldown studies and immunoprecipitation assays were performed to validate the identified yeast two-hybrid interaction between Ku and BRG1. (A) Schematic representation of the Ku dimer with the putative BRG1 interaction surface indicated. Ku86 is shown in green and Ku70 displayed in blue and red. The amino acid residues 425 to 660 of Ku70, marked in red, were found to associate with the HSA-BRK region of BRG1. (B) A purified BRG1 fragment containing the HSA and BRK (aa 437 to 678) domains interacts with a purified Ku70 fragment (aa 450 to 537). Bacterially expressed MBP-BRG1 and GST-Ku proteins were used for glutathione-Sepharose pulldown analysis. Stained SDS-PAGE shows that the BRG1 protein associates with the Ku70 fragment. Mass spectrometry analysis confirms the identity of BRG1, Ku70, or nonspecific bands. (C) Coimmunoprecipitation assays were performed using whole-cell lysates from parental SW-13 cells, expressing BRG1-3×Flag and Ku70-V5 proteins, and antibodies specific to the indicated epitope tag. Immunopurified complexes were resolved by 7.5% SDS-PAGE and analyzed by Western blotting (IB) using anti-BRG1 or -Ku70 antibodies. (D) Ku70 associates with BRG1 in various cell types. Immunoprecipitation assays were performed using whole-cell lysates IPs were performed as described above. Immunopurified complexes were washed, resolved by SDS-PAGE, and analyzed by Western blotting using Ku70-specific antibody. (E) Complexes containing endogenous Ku and BRG1 were immunoprecipitated from transfected SW-13 cells expressing BRG1, using antibodies specific for SWI/SNF subunit BRG1, BAF250a, BAF170 or BAF155, as well as for Ku70 or Ku86. Immunopurified proteins were resolved by SDS-PAGE and immunoblotted using BRG1, Ku70, Ku86, BAF250a, BAF170, or BAF155 antibodies. (F) The HSA domain of BRG1 is required for Ku binding. Whole-cell lysates from SW-13 cells expressing BRG1 or mutant BRG1 proteins ΔHSA, or ΔBRK were immunoprecipitated, in the presence of benzonase nuclease, using antibodies specific for BRG1 or Ku70. Purified proteins were resolved by SDS-PAGE and analyzed by immunoblotting with anti-BRG1 antibody. For each immunoprecipitation, input represents 10% of the total lysate used and normal IgG was used to control for nonspecific interactions.