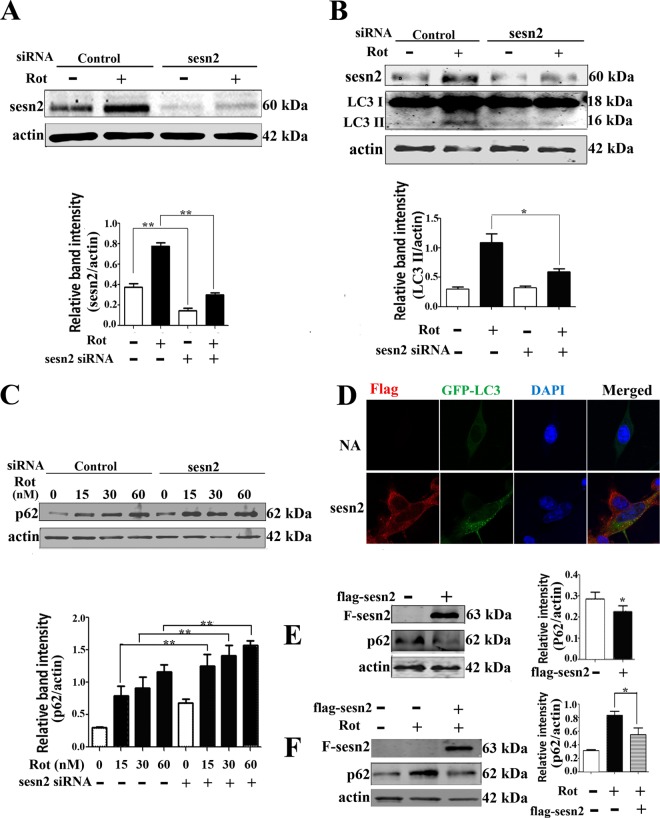
FIG 3.
Sestrin2 upregulation contributed to autophagy enhancement. (A) Sestrin2 was knocked down by transient transfection with sestrin2 siRNA for 48 h. Control cells were transfected with control siRNA. After sestrin2 knockdown, cells were treated with 20 nM rotenone and immunoblotted for sestrin2 protein. (B and C) Sestrin2 knockdown cells and control cells were treated with 20 nM rotenone and immunoblotted for LC3 (B) and p62 (C). (D) Florescent detection of LC3 (green) after cotransfecting Flag-sestrin2 plasmids (250 ng/well) with pcDNA4 GFP-LC3 plasmids for 48 h. Cells treated with equal amounts of empty Flag plasmids but no Flag-sestrin2 plasmids were used as controls. Spotted distribution of GFP indicated enhanced autophagy activity. DAPI, 4′,6-diamidino-2-phenylindole. NA, negative control. (E) Cells were transiently transfected with control or Flag-sestrin2 plasmids for 48 h, and Flag and p62 protein levels were detected. (F) Cells were transfected with control or Flag-sestrin2 plasmids for 48 h and then treated with rotenone for an additional 12 h for detection of a change in p62 protein levels. Actin was used as a loading control. Protein band density of p62 relative to that of actin was analyzed by densitometry. Values are the means ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01.