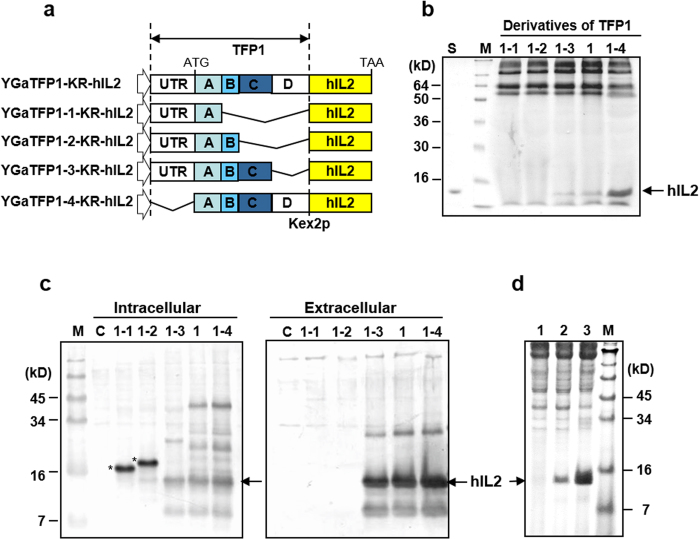
Figure 2. Effects of the translational fusion partner (TFP) 1 domains on the secretion of a rarely secreted protein, human interleukin (hIL)-2.
(a) Schematic diagram of TFP1 derivatives. TFP1 was divided into five domains (UTR: 5′ untranslated region, A: signal sequence, B: N-glycosylation site, C: Ser and Ala rich sequence, D: a flanking sequence). Deleted domains indicated by the bent line. (b) SDS-PAGE analysis of hIL-2 secreted into the culture medium by recombinant strains carrying the plasmids harbouring TFP1 derivatives. Lane S: recombinant hIL-2 produced by Escherichia coli. M: standard protein size marker. The protein is revealed by Coomassie staining. (c) Western blot analysis of intracellular and extracellular proteins produced by recombinant strains carrying the indicated plasmids. C: host strain carrying the mock vector. (d) SDS-PAGE followed by Coomassie staining for the comparison of hIL-2 secretion using different signal sequences. Lane 1: α-amylase signal peptide from B. subtilis, lane 2: prepro signal peptide of mating factor α from S. cerevisiae, and lane 3: TFP1-4 in this study.