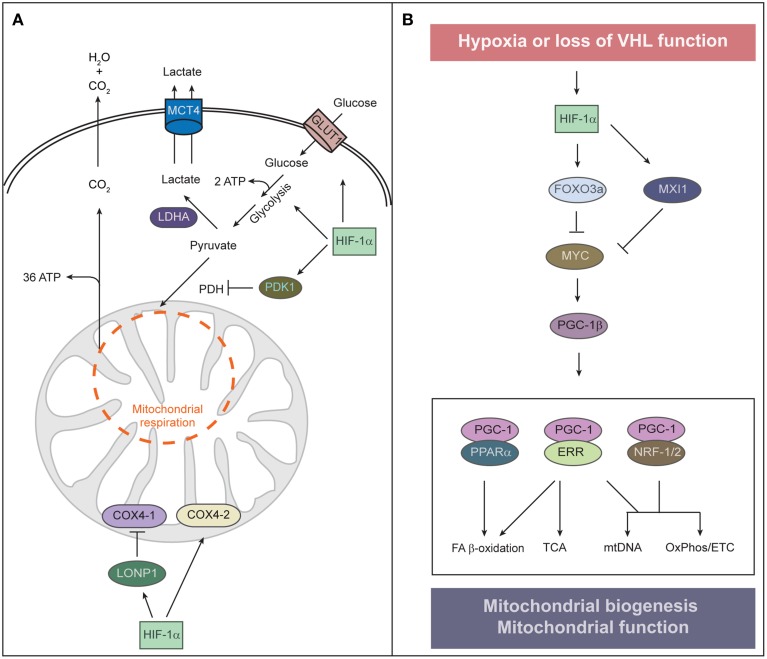
Figure 2.
Regulation of mitochondrial function and abundance by HIF-α. (A) To adapt to low oxygen tension, cells undergo two HIF-1α-mediated alterations of cellular metabolism: O2-independent ATP production and reduction of mitochondrial O2 consumption. HIF-1α signaling also contributes to the Warburg effect of aerobic glycolysis—that is, an uncoupling of glycolysis from O2 levels—by stimulating the expression of the glucose transporter GLUT1 and glycolytic enzymes. Increased glycolysis generates increased levels of pyruvate, which is largely converted to lactate by HIF-inducible lactate dehydrogenase A (LDHA) and removed from the cell by the monocarboxylate transporter 4 (MCT4). HIF-1α induces pyruvate dehydrogenase kinase 1 (PDK1), which inhibits pyruvate dehydrogenase (PDH) and blocks conversion of pyruvate to acetyl-CoA, resulting in decreased flux through the tricarboxylic acid (TCA) cycle. Decreased TCA cycle activity attenuates oxidative phosphorylation and excessive mitochondrial ROS production. Under normoxia, COX4-1 is the predominant isoform of COX4 present in complex IV of the electron transport chain, which transfers electrons to O2. Under hypoxia, HIF-1α upregulates the expression of COX4-2 and the mitochondrial protease LONP1, which in turn degrades COX4-1. COX4-2 is more efficient at facilitating the electron transfer to O2 and thereby protects the cell from oxidative damage during hypoxia. (B) Control of mitochondrial biogenesis by HIF-α. HIF-1α induces the expression of MAX-interacting protein 1 (MXI1), a repressor of MYC activity, and thereby represses a subset of MYC target genes such as PGC-1β. HIF-1α-dependent activation of FOXO3a inhibits MYC activity by reducing MYC protein stability. Interaction between PGC-1 and transcription factors such as PPARα, ERR, and NRF-1/2 orchestrates the major functions of mitochondria. HIF-1α-mediated inhibition of MYC and PGC-1 results in reduced mitochondrial biogenesis.