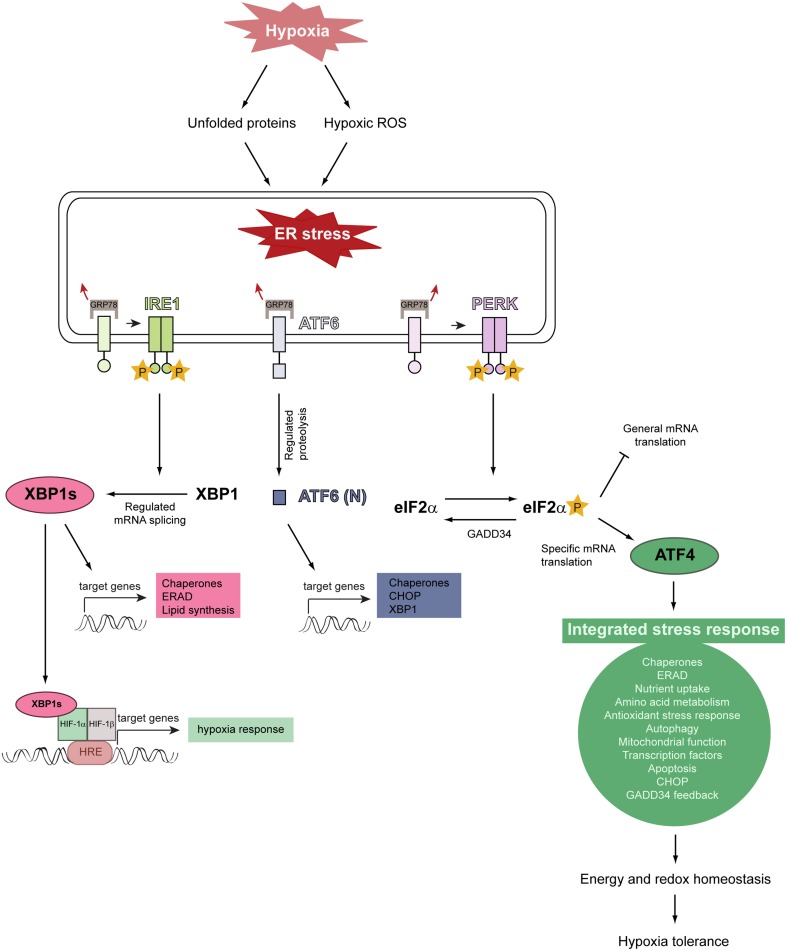
Figure 4.
Model illustrating the relationship between hypoxia, ER stress, and activation of the unfolded protein response. Severe hypoxic stress perturbs and reduces O2-dependent protein folding capacity, resulting in the accumulation and aggregation of misfolded proteins in the ER lumen. Hypoxia increases intracellular ROS production and ROS stimulate multiple biological responses during O2 deprivation. Unfolded proteins and hypoxic ROS trigger ER stress which leads to HIF-α-independent activation of the UPR. The UPR is initiated by the stress-sensing ER transmembrane proteins PERK, IRE1, and ATF6. The chaperone GRP78 is normally bound to these ER stress sensors and keeps them inactive, but dissociates from them under ER stress conditions. This dissociation leads to the activation of the three UPR pathways. GRP78 dissociation allows PERK to homodimerize, which facilitates autotransphosphorylation and kinase domain activation. Activated PERK phosphorylates eIF2α which decreases general translation while increasing the preferential translation of specific proteins, such as the transcription factor ATF4. ATF4 triggers the activation of a gene expression program referred to as the integrated stress response. ATF4 induces the expression of GADD34, which acts as a negative regulator of the PERK pathway by dephosphorylating eIF2α. The integrated stress response promotes energy and redox homeostasis and is an important prosurvival mechanism under moderate hypoxia. IRE1 homodimerization, followed by autotransphosphorylation, triggers its RNase activity. IRE1-mediated splicing of full-length XBP1 mRNA generates XBP1s, which encodes an active transcription factor. ER stress leads to the translocation of ATF6 to the Golgi, where it is cleaved by regulated intramembrane proteolysis to produce the active transcription factor. Modified from Faust and Kovacs (2014).