Figure 1. TRPV1 tetrameric concatemer mimics the activation profile of the wild-type protein.
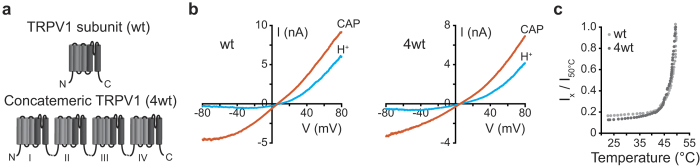
(a) Schematic representation of wt and 4wt TRPV1 constructs. The concatemeric rat TRPV1 (rTRPV1) construct was engineered by insertion of a unique restriction enzyme site at the C terminal (without the stop codon) and to the N-terminal (without the start codon) of adjacent wt subunits (bottom; dashed line). (b) Both capsaicin (CAP; 1 μM; orange line) and extracellular protons (H+; pH 5.5; cyan line) elicited robust, outwardly rectifying currents in HEK293 cells transiently expressing either rTRPV1 (“wt”; left) or concatemeric rTRPV1 (four subunits, “4wt”; right) construct. Current-voltage relationship traces were recorded using whole-cell patch-clamp recording (in 1 s−1 voltage-ramps between −80 and +80 mV). (c) Thermal response profiles of oocytes expressing either rTRPV1 (“wt”; light grey) or concatemeric rTRPV1 (“4wt”; dark grey) construct. The current at each indicated temperature was normalized to that evoked at 50 °C. Temperature threshold for both wt and 4wt constructs was 43 °C; the Q10 was 27 for wt and 26 for 4wt rTRPV1.
