Figure 4. A single VBS-containing subunit is sufficient to evoke capsaicin sensitive current.
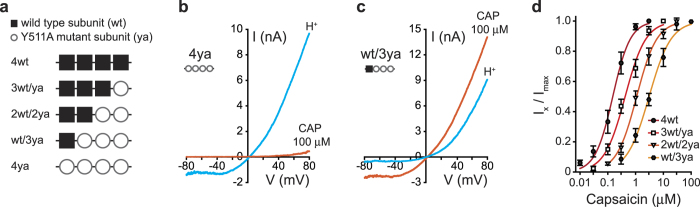
(a) Schematic representation of the different tetrameric concatemeric constructs used to study the stoichiometry for TRPV1 activation by capsaicin. Black squares represent wt subunits while empty circles represent subunits with an Y511A mutated VBS (ya). (b, c) Current-voltage relationship traces in HEK293 cells transiently expressing the 4ya (b) and wt/3ya (c) constructs in response to capsaicin (CAP; 100 μM; orange line) and protons (H+; pH 5.5; cyan line). Currents were recorded using whole-cell patch-clamp recording (in 1 s−1 voltage ramps between −80 and +80 mV). Mutations in all VBS nearly eliminated the capsaicin response, while the proton response was intact (b). A single intact VBS was sufficient to produce a robust capsaicin response (c). (d) Normalized concentration-response relationships for capsaicin of the different concatemers. Each point represents the average (±SEM) response of 10–14 HEK293 cells transiently expressing the respective construct. Solid lines are fit to the Hill equation (see Eq. (2)): 4wt (full circles, dark red line; nH = 1.4; EC50 = 0.15 ± 0.02 μM), 3wt/ya (empty squares, red line; nH = 1.1; EC50 = 0.42 ± 0.07 μM), 2wt/2ya (empty triangle; orange line; nH = 1.2; EC50 = 1.09 ± 0.08 μM) and wt/3ya (full diamonds, yellow line; nH = 1.1; EC50 = 3.10 ± 0.47 μM). Reduction in the number of subunits containing an intact VBS leads to a shift in the affinity of capsaicin.
