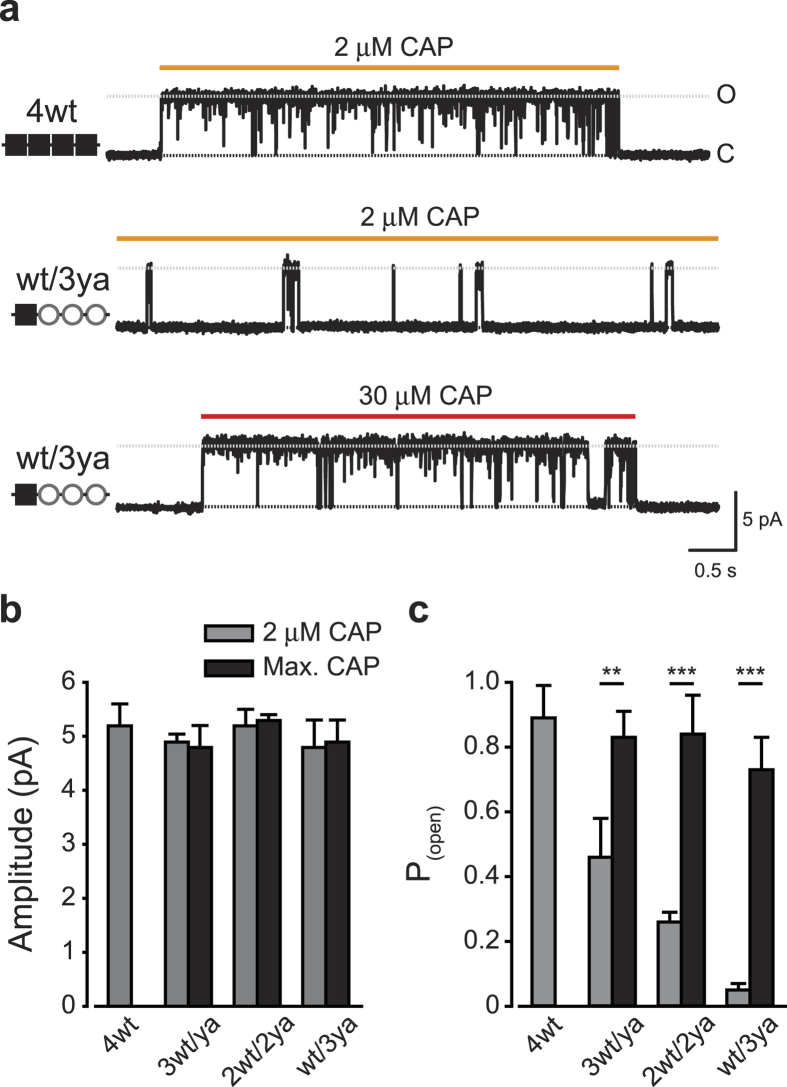
Figure 6. A single VBS bound subunit evokes full channel activation.
(a) Representative current records from an excised outside-out membrane patch of capsaicin-exposed Flp-in TREX HEK293 cells stably transfected with 4wt and wt/3ya TRPV1 concatemers. Upward (outward) currents indicate channel opening (grey dash line). Shown are representative channel activities upon exposing the patches to saturating capsaicin concentration (2 μM CAP; orange line) of 4wt (top trace) and wt/3ya (middle trace) concatemers. Also shown is a representative channel activity of the same wt/3ya patch exposed to 30 μM capsaicin (30 μM CAP; red line; bottom trace). Holding potential at +60 mV sampled at 50 kHz and filtered at 1 kHz for display. Note the similar channel activation for the relative saturating capsaicin concentration of the two constructs. (b, c) Bar diagram representing the average (±SEM) amplitude (b) and open probability (P(open); (c) of the single-channel current activated by capsaicin at 2 μM (grey bars) and saturating concentration (Max. CAP; black bars) of the various concatemeric constructs. Each bar represents an average of 3–5 patches. The statistical significance between the 2 μM and the relative saturating concentration of capsaicin was determined using paired Student’s t test, where **represents P ≤ 0.01 and ***represents P ≤ 0.001. Note that all the different VBS-mutated concatemeric constructs reached similar open probability to that reached by the 4wt construct at saturating capsaicin concentrations.