Abstract
Neurocutaneous melanosis (NCM) is a rare condition characterized by central nervous system melanocytic tumors associated with congenital melanocytic nevi. Phacomatosis pigmentovascularis (PPV) is an association of vascular nevus with pigmentary nevus. Aberrant maturation of neural crest-derived cells is considered to be related to pathogenesis in both conditions. However, association of NCM and PPV has not been reported to the best of our knowledge. Melanocytoma, which usually involves the leptomeninges or spinal cord, is extremely rare in the retroperitoneum. We present here a case of a patient with NCM, PPV, and melanocytic tumors in the spinal cord and retroperitoneum, which were treated surgically. A 40-year-old woman had a 2-year history of dysesthesia and weakness in the left leg. History included congenital giant blue nevus-like lesion in the trunk, a port-wine stain in the sacral area, and Caesarean section performed 8 years before, when diffuse pigmentation in the peritoneum was noted. Magnetic resonance (MR) imaging of the spine revealed an intramedullary tumor at T10 level with paramagnetic signal characteristics. The spinal cord tumor was totally removed, and the histological diagnosis was melanocytoma. Three months later, a left retroperitoneal mass with histological features of melanocytic tumor was removed. Neither tumors recurred and the patient stays ambulatory 4 years after the surgery. Multiple subtypes of melanocytic tumors with distinctive features of NCM and PPV can develop simultaneously, mimicking malignant melanoma. Gross total resection of each tumor, when indicated, is beneficial.
Keywords: phacomatosis pigmentovascularis, neurocutaneous melanosis, intramedullary melanocytoma, retroperitoneal tumor
Introduction
Neurocutaneous melanosis (NCM) is a rare condition characterized by melanocytic tumors of the central nervous system coexisting with giant congenital melanocytic nevi.5) Its prognosis is ominous due to high incidence of melanoma.11) Phacomatosis pigmentovascularis (PPV), another rare condition, is an association of widespread vascular nevus with extensive pigmentary nevus.9) Patients with PPV may have brain lesions similar to those of Sturge–Weber syndrome, such as meningeal angiomatous malformation and cortical atrophy and/or calcification, but not melanocytic tumor. To the best of our knowledge, association of NCM and PPV has not been described in the literature.
Melanocytoma is a benign tumor that usually arises in the spinal canal or posterior fossa leptomeninges15,16,25) and rarely in the spinal cord.6) Melanocytoma in the retroperitoneum is very rare and only one case has been reported.18)
In this report, a case of a patient with concurrent development of NCM and PPV with melanocytic tumors in the spinal cord and retroperitoneum is presented.
Case Report
A 40-year-old woman presented with a 2-year history of tingling dysesthesia in the left lower extremity and 2-month history of mild foot drop on the left. She had a giant congenital nevus in the trunk which had been stable for years and left unexamined. She had undergone Caesarian section at the age of 32, and her obstetrician noted diffusely pigmented peritoneum. Family history was negative for traits of systemic pigmentous diseases.
On physical examination, there were large blue lesions on the trunk and a port-wine stain in the sacral area. Examinations of the cranial nerves and motor and sensory functions in the four extremities were intact except for positive Romberg sign and dysesthesia in the left L5 dermatome. Deep tendon reflexes were normal in the upper extremities and hyperactive in the lower extremities.
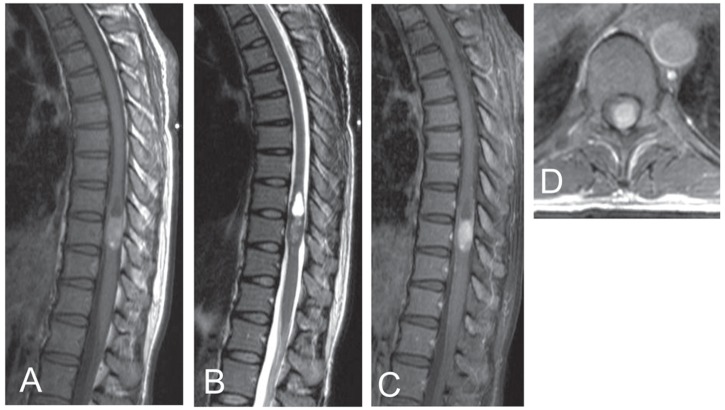
Magnetic resonance (MR) imaging of the thoracic spine showed a partially cystic intramedullary mass at T10 level, which was hyperintense on T1- and hypointense on T2-weighted images, denoting a paramagnetic character. It enhanced homogeneously with gadolinium diethylpentoic acid (Fig. 1). MR imaging of the brain, cervical, and lumbar spine was negative.
Fig. 1.
Magnetic resonance (MR) imaging of the thoracic spine showing a partially cystic intramedullary mass, which is hyperintense on T1-weighted image (A), hypointense on T2-weighted image (B), and enhances homogenously with gadolinium diethylpentoic acid (C). Contrast-enhanced T1-weighted axial image shows displacement of the normal parenchyma of the cord ventrally (D).
In surgery, myoarchitectonic spinolaminoplasty13) from T9 through T11 was done. The skin over the lesion was normal, but the laminae and dura had multiple pigmentations. The black-colored tumor was observed on the dorsal surface of the cord, with a soft pial part that was easily peeled off and a elastic hard intramedullary part that was adherent to the pia and richly vascularized (Fig. 2). The tumor was totally removed through a midline myelotomy. Postoperatively, the patient had dysesthesia in the abdomen and lower extremities. Joint position sense was diminished in the lower extremities, more prominently on the left. Pain and temperature sensation was temporarily disturbed in the right lower extremity. The muscle strength was unaffected. The patient became ambulatory in 2 weeks.
Fig. 2.
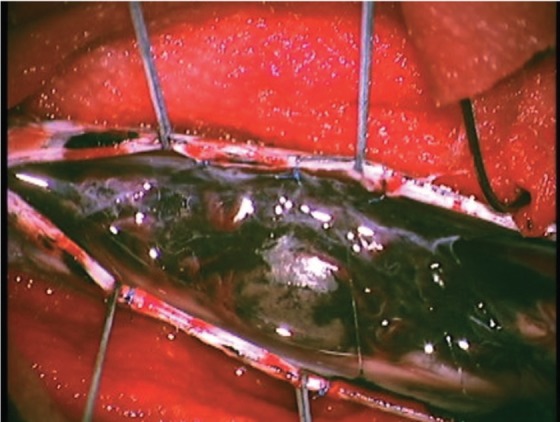
Intraoperative photograph shows pigmentations in the dura and arachnoid. Black tumor is seen on the posterior surface of the cord.
Three months later, the patient had computed tomography of the abdomen for a complaint of persistent abdominal dysesthesia. A low-dense, nonenhancing 4-cm mass was found in the left retroperitoneum displacing the pancreas. The lesion was hyperintense on T2-weighted MR images (Fig. 3) and manifested a high uptake of 2-[(18) F]-fluoro-2-deoxy-D-glucose (FDG) on positron emission tomography (PET). The pigmented tumor in the retroperitoneal adipose tissue was totally removed through a conventional laparotomy without adverse consequences.
Fig. 3.
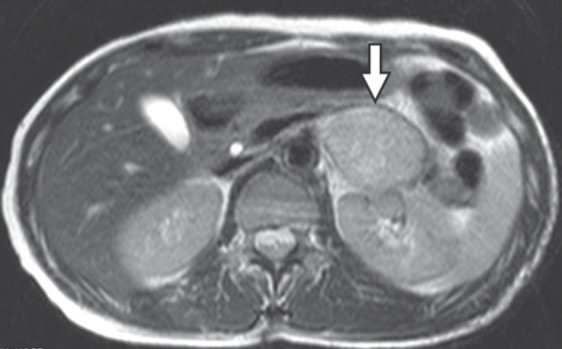
The T2-weighted magnetic resonance (MR) image of the abdomen shows a round mass (arrow) in the retroperitoneum.
Presently, 4 years after the operations, the patient ambulates freely with residual dysesthesia in the lower abdomen and lower extremities. Thoracic spine MR imaging and whole-body FDG-PET scan reveal no tumor recurrence (Fig. 4).
Fig. 4.
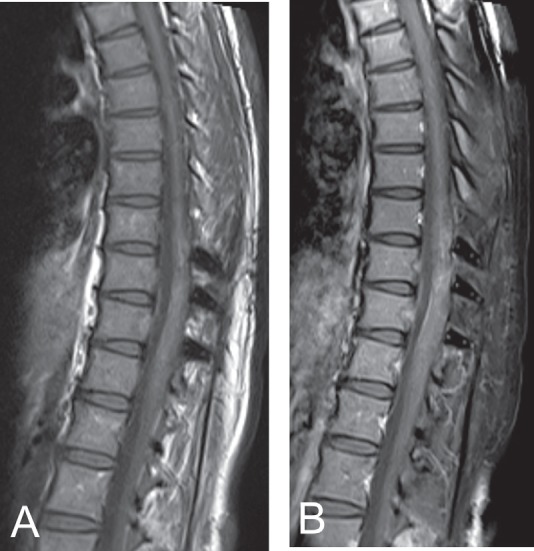
Magnetic resonance (MR) imaging of the thoracic spine 4 years after surgery. T1-weighted images without contrast (A) and with contrast (B) show no tumor recurrence.
Histology of the spinal cord tumor showed dense proliferation of polyhedral cells with round to ovoid nucleus and faintly eosinophilic cytoplasm containing melanin, without necrosis. Mitosis was not evident. Soft part consisted of monotonous proliferation of cells, and firm portion had a background feature of fibrous tissue, resembling dermal nevus. The tumor cells infiltrated into the perivascular space and spinal cord parenchyma (Fig. 5). The cells reacted positive for HMB-45. Index ratio of Ki-67/MIB-1-positive cells was less than 5%. The diagnosis was melanocytoma.
Fig. 5.
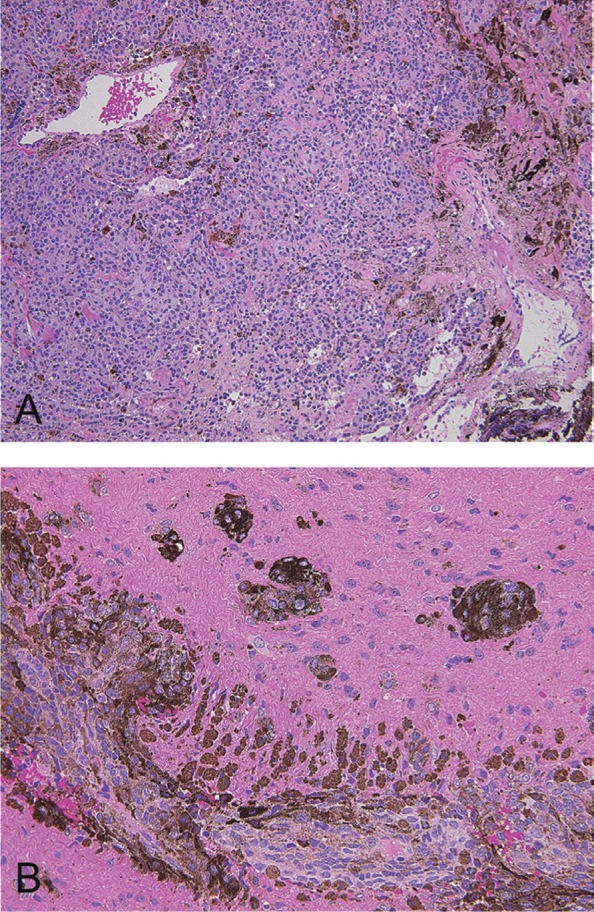
Hematoxylin and eosin-stained section of the intramedullary tumor shows proliferation of cells with fibrous tissue background (A). The tumor infiltrates into the spinal cord parenchyme (B).
Histology of the skin biopsy specimen from the back revealed deposition of melanin, fibrosis, and proliferation of melanophages and melanocytes in the middle to lower dermis and subcutaneous tissue. The finding was consistent with a diagnosis of blue nevus.
The retroperitoneal tumor consisted of diffuse proliferation of ovoid and spindle cells containing melanin. There was no necrosis and rarely mitosis. Immunohistochemical stain was positive for HMB-45, C-KIT, and S-100 and negative for smooth muscle actin, cytokeratin (AE1/3), CD56, TUJ-1, and CD99. Proliferative index by Ki-67/MIB-1 was 5%. The diagnosis was melanocytic tumor with questionable malignant property.
Discussion
This patient had congenital melanocytic nevus and intramedullary melanocytoma, meeting the diagnostic criteria for NCM.5) Concurrently she had a vascular nevus, also compatible with a diagnosis of PPV.8) The pathogenesis of NCM is postulated to be a congenital error in morphogenesis of the neuroectoderm and/or neural crest.11) The pathogenesis of PPV is presumed to be an aberrant proliferation of angioblasts and melanoblasts, or vasomotor nerve cells derived from the neural crest.9,21) The coexistence of NCM and PPV in this case suggests that they may have a common cause in the embryonal differentiations in the neural crest.
Patients with NCM typically present in the first decade of life, and the mortality is 54% within 3 years of onset due to development of melanoma or leptomeningeal melanosis.5,11) These studies probably had selection bias because they were based on autopsy series. In a recent study, of 205 patients with large congenital melanocytic nevi taking MR imaging survey, 8 patients had asymptomatic and 9 had symptomatic NCM.7) In a 5-year follow-up study concerning asymptomatic NCM patients, 1 out of 10 developed neurological symptom.4) The presented case may exemplify a future status of patients with asymptomatic NCM.
MR imaging was critical for diagnosing the spinal cord melanocytoma, which exhibited high T1- and low T2 signal intensity. Such paramagnetic signal is characteristic to melanocytic tumors with high melanin contents, but not to those with low melanin contents.17,24) The high T2 signal exhibited by the retroperitoneal tumor may be caused by lower melanin concentration.
Histology of the spinal cord tumor revealed proliferation of melanocytes with immunoreactivity for HMB-45 and Ki-67/MIB-1 proliferation index no more than 5%.2) Infiltration of the neural parenchyme is a common finding and does not indicate a malignancy.6) Features of melanoma such as cellular atypia, mitosis, necrosis, hemorrhage, or host responses were absent in this case.22)
Recurrence of intramedullary melanocytoma is rare after total excision.3,6,12,23) Radiation therapy with dosage greater than 45 Gy is recommended when total resection is not achieved20) because partial resection may result in recurrence and malignant transformation.1,10,17,19,25)
Only one case of retroperitoneal melanocytic tumor is reported, of a 5-month-old boy with a posterior fossa meningeal melanocytoma and tumors in the surrenal glands and renal capsule, which remained stable for 10 months without treatment.18) In our experience, total resection of the retroperitoneal melanocytic tumor resulted in good outcome, but further studies are needed to provide a recommendation of treatment.
When a patient with congenital nevus presents with tumors in the central nervous system and retroperitoneum, it may be mistaken for advanced melanoma with metastasis, for which surgery is not recommended. However, this case indicates that surgery is a reasonable option for multiple melanocytic tumors in a patient with NCM and PPV, analogous to tumors in neurofibromatosis, a neurocristopathy familiar to neurosurgeons.14)
References
- 1). Barth A, Pizzolato GP, Berney J: [Intramedullary meningeal melanocytoma]. Neurochirurgie 39: 188– 194, 1993. (French) [PubMed] [Google Scholar]
- 2). Brat DJ, Giannini C, Scheithauer BW, Burger PC: Primary melanocytic neoplasms of the central nervous systems. Am J Surg Pathol 23: 745– 754, 1999. [DOI] [PubMed] [Google Scholar]
- 3). Chacko G, Rajshekhar V: Thoracic intramedullary melanocytoma with long-term follow-up. J Neurosurg Spine 9: 589– 592, 2008. [DOI] [PubMed] [Google Scholar]
- 4). Foster RD, Williams ML, Barkovich AJ, Hoffman WY, Mathes SJ, Frieden IJ: Giant congenital melanocytic nevi: the significance of neurocutaneous melanosis in neurologically asymptomatic children. Plast Reconstr Surg 107: 933– 941, 2001. [DOI] [PubMed] [Google Scholar]
- 5). Fox H: Neurocutaneous melanosis, in Vinken PJ, Bruyn GW. (eds): Handbook of Clinical Neurology. Amsterdam, North Holland, 1972, pp 414– 428 [Google Scholar]
- 6). Glick R, Baker C, Husain S, Hays A, Hibshoosh H: Primary melanocytomas of the spinal cord: a report of seven cases. Clin Neuropathol 16: 127– 132, 1997. [PubMed] [Google Scholar]
- 7). Hale EK, Stein J, Ben-Porat L, Panageas KS, Eichenbaum MS, Marghoob AA, Osman I, Kopf AW, Polsky D: Association of melanoma and neurocutaneous melanocytosis with large congenital melanocytic naevi—results from the NYU-LCMN registry. Br J Dermatol 152: 512– 517, 2005. [DOI] [PubMed] [Google Scholar]
- 8). Happle R: Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol 141: 385– 388, 2005. [DOI] [PubMed] [Google Scholar]
- 9). Hasegawa Y, Yasuhara M: Phakomatosis pigmentovascularis type IVa. Arch Dermatol 121: 651– 655, 1985. [PubMed] [Google Scholar]
- 10). Horn EM, Nakaji P, Coons SW, Dickman CA: Surgical treatment for intramedullary spinal cord melanocytomas. J Neurosurg Spine 9: 48– 54, 2008. [DOI] [PubMed] [Google Scholar]
- 11). Kadonaga JN, Frieden IJ: Neurocutaneous melanosis: definition and review of the literature. J Am Acad Dermatol 24: 747– 755, 1991. [DOI] [PubMed] [Google Scholar]
- 12). Karikari IO, Powers CJ, Bagley CA, Cummings TJ, Radhakrishnan S, Friedman AH: Primary intramedullary melanocytoma of the spinal cord: case report. Neurosurgery 64: E777– 778; discussion E778, 2009. [DOI] [PubMed] [Google Scholar]
- 13). Kim P, Murata H, Kurokawa R, Takaishi Y, Asakuno K, Kawamoto T: Myoarchitectonic spinolaminoplasty: efficacy in reconstituting the cervical musculature and preserving biomechanical function. J Neurosurg Spine 7: 293– 304, 2007. [DOI] [PubMed] [Google Scholar]
- 14). Lakkis MM, Tennekoon GI: Neurofibromatosis type 1. I. General overview. J Neurosci Res 62: 755– 763, 2000. [DOI] [PubMed] [Google Scholar]
- 15). Limas C, Tio FO: Meningeal melanocytoma (“melanotic meningioma”). Its melanocytic origin as revealed by electron microscopy. Cancer 30: 1286– 1294, 1972. [DOI] [PubMed] [Google Scholar]
- 16). Litofsky NS, Zee CS, Breeze RE, Chandrasoma PT: Meningeal melanocytoma: diagnostic criteria for a rare lesion. Neurosurgery 31: 945– 948, 1992. [DOI] [PubMed] [Google Scholar]
- 17). Ochiai H, Nakano S, Miyahara S, Goya T, Wakisaka S, Kinoshita K: Magnetic resonance imaging of a malignant transformation of an intracranial cellular blue nevus. A case report. Surg Neurol 37: 371– 373, 1992. [DOI] [PubMed] [Google Scholar]
- 18). Oruckaptan HH, Soylemezoglu F, Kutluk T, Akalan N: Benign melanocytic tumor in infancy: discussion on a rare case and review of the literature. Pediatr Neurosurg 32: 240– 247, 2000. [DOI] [PubMed] [Google Scholar]
- 19). Perrini P, Caniglia M, Pieroni M, Castagna M, Parenti GF: Malignant transformation of intramedullary melanocytoma: case report. Neurosurgery 67: E867– 869; discussion E869, 2010. [DOI] [PubMed] [Google Scholar]
- 20). Rades D, Schild SE, Tatagiba M, Molina HA, Alberti W: Therapy of meningeal melanocytomas. Cancer 100: 2442– 2447, 2004. [DOI] [PubMed] [Google Scholar]
- 21). Ruiz-Maldonado R, Tamayo L, Laterza AM, Brawn G, Lopez A: Phacomatosis pigmentovascularis: a new syndrome? Report of four cases. Pediatr Dermatol 4: 189– 196, 1987. [DOI] [PubMed] [Google Scholar]
- 22). Scolyer RA, Zhuang L, Palmer AA, Thompson JF, McCarthy SW: Combined naevus: a benign lesion frequently misdiagnosed both clinically and pathologically as melanoma. Pathology 36: 419– 427, 2004. [DOI] [PubMed] [Google Scholar]
- 23). Takenaka N, Imanishi T, Kondoh A, Ohnumata A, Fukuda J, Yagishita S: [Primary intramedullary melanocytoma of medulla oblongata: a case report]. No Shinkei Geka 24: 247– 252, 1996. (Japanese) [PubMed] [Google Scholar]
- 24). Uematsu Y, Yukawa S, Yokote H, Itakura T, Hayashi S, Komai N: Meningeal melanocytoma: magnetic resonance imaging characteristics and pathological features. Case report. J Neurosurg 76: 705– 709, 1992. [DOI] [PubMed] [Google Scholar]
- 25). Winston KR, Sotrel A, Schnitt SJ: Meningeal melanocytoma. Case report and review of the clinical and histological features. J Neurosurg 66: 50– 57, 1987. [DOI] [PubMed] [Google Scholar]