Abstract
Background: To describe the clinical manifestations and ancillary examination outcomes of ocular syphilis in Southeast China. Materials and methods: This is a retrospective, nonrandom case study. Demographic information, serum and cerebrospinal fluid (CSF) test results, and findings of fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA), and spectral domain optical coherence tomography (SD-OCT) were analyzed. Results: The study examined 21 eyes of 13 patients (average age 50.3 ± 5.9 (range 37-61) years). HIV co-infection was found in one patient. The most common manifestation was chorioretinitis (52.4%). Disc hyperfluorescence (66.7%) and persistent dark spots (91.7%) were the most common findings on FFA and ICGA, respectively. The inner segment/outer segment junction (IS/OS) loss was the most frequent manifestation (86.7%). Among the six patients with confirmed neurosyphilis, the average CSF protein level was 528.8 ± 327.1 mg/L. Visual acuity (VA) was improved in 8 of 13 eyes (61.5%) after treatment. Conclusions: The manifestations of ocular syphilis can mimic any eye disease. Chorioretinitis was the most common finding in this case series. “Leopard spots” was the characteristic manifestation on FFA. IS/OS loss was the most common finding in patients with posterior uveitis on SD-OCT. Lumbar puncture can contribute to the diagnosis of neurosyphilis. Treatment for ocular syphilis was effective in these patients.
Keywords: Chorioretinitis, CSF, FFA, ICGA, neurosyphilis, ocular syphilis, syphilis, SD-OCT
Introduction
After nearly vanishing with the use of antibiotics, the incidence of syphilis has increased worldwide since the year 2000. Resurgent syphilis epidemics have been reported in a number of developed countries, including the UK and US [1,2]. However, 90% of new syphilis cases are in developing countries [3]. In China, the incidence of primary and secondary syphilis was 5.67 cases per 100,000 in 2005, which is substantially higher than that in most developed countries [4]. Syphilis is an infectious disease caused by the spirochete Treponema pallidum, which can cause morbidity in almost any part of the body [5]. If left untreated, syphilis can progress through four stages: primary, secondary, latent, and tertiary. Ocular involvement in syphilis is not uncommon, and it can develop at any stage of the disease.
Ocular syphilis can mimic a variety of eye diseases, including interstitial keratitis; anterior, intermediate, and posterior uveitis; chorioretinitis; retinitis; retinal vasculitis; and cranial nerve and optic neuropathies [6]. Thus, it is termed the great masquerader. In recent years, ocular syphilis has been reported in numerous developed countries, and uveitis is the most common manifestation of syphilis in the eye [7-9]. In Asia, scholars in Singapore and Japan have recently reported several cases of ocular syphilis [10-12]. However, in Southeast China, which has one the highest incidence rates of syphilis [4], ocular syphilis has rarely been reported. Here, we report a small series of cases of ocular syphilis, and we describe the clinical manifestations and ancillary examination outcomes of ocular syphilis in Southeast China.
Materials and methods
Patients diagnosed with ocular syphilis at the Affiliated Sir Run Run Shaw Hospital of Zhejiang University between May of 2009 and April of 2014 were reviewed retrospectively. This retrospective study was approved by the Ethics Committee of the Affiliated Sir Run Run Shaw Hospital of Zhejiang University and followed the tenets of the Declaration of Helsinki. Written informed consent was not required because data were analyzed anonymously and retrospectively. Verbal informed consent was obtained from the patients, and this was sufficient for approval by the Ethics Committee of the Affiliated Sir Run Run Shaw Hospital of Zhejiang University. A summary of the patients’ demographic information was prepared from the patients’ records. The diagnosis of ocular syphilis was based on the results of non-treponemal (rapid plasma reagin, RPR) and treponemal (T. pallidum particle agglutination, TPPA) serological tests and on ocular inflammation that could not be attributed to any other eye disease. The diagnosis of neurosyphilis was made according to criteria reported previously [13].
The patients underwent a comprehensive ophthalmologic examination that included testing of the best-corrected visual acuity (BCVA), non-contact intraocular pressure, visual field, slit-lamp examination of the anterior and posterior segments, fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA) (Heidelberg Engineering Retina Angiograph; Heidelberg Engineering, Carlsbad, CA, USA), spectral domain optical coherence tomography (SD-OCT; Carl Zeiss Meditec, Dublin, CA, USA), and fundus photography (Topcon, Japan). A lumbar puncture was performed according to the recommendation of the Centers for Disease Control and Prevention [14]. Other systemic laboratory (ancillary) tests were reviewed.
The recorded angiographic patterns on FFA included retinal staining/capillary leakage, vascular staining, macular edema, disc hyperfluorescence [15,16]. The recorded angiographic patterns on ICGA included fuzzy choroidal vessels, persistent dark dots, and hot dots [16]. BCVA was evaluated using a standard logarithmic visual acuity chart. A change of two lines indicated visual improvement or worsening.
Results
Data for 21 eyes of 13 patients with ocular syphilis were analyzed retrospectively. The average age of the patients was 50.3 ± 5.9 (range 37-61) years. Eight patients (61.5%) had bilateral involvement, while five (46.2%) had unilateral involvement. Of the 13 patients, 7 (53.8%) were male and 6 (42.9%) were female (Table 1). All patients denied being homosexuality. Eleven patients had ocular involvement as the initial manifestation, while one had been diagnosed with neurosyphilis and the other had simultaneous eye and ear involvements and was also HIV positive. The most common complaint was blurring of vision (11 of 13 patients). There was one patient (7.7%) with simultaneous essential hypertension, two (15.4%) with type II diabetes mellitus, one with multiple sclerosis, one (7.7%) with lupus nephritis, one (7.7%) with a positive serum test for HLA-B27, and one patient with a human immunodeficiency virus (HIV) infection (7.7%) (Table 1). The median follow-up period was 4.1 ± 5.8 (range 1-22) months.
Table 1.
Demographic characteristics, treatment results of all subjects
| Age (y)/Gender | Presenting complaint | Ocular diagnosis | Systemic disease | Treatment | Initial BCVA (R/L) | Final BCVA (R/L) | Follow-up (m) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 52/M | OU blurred vision | OU retrobulbar neuritis | MS | UR | 0.1/0.08 | UR | 2 |
| 2 | 49/M | OD blurred vision | OU choroidoretinitis | - | Pen 24 MUI iv d for 2 w; BPen, 2.4 MUI im d for 3 w | 0.3/0.6 | UR | 4 |
| 3 | 61/F | OU Blurred vision | OU choroidoretinitis; OS BRVO; OS VH | DM | Pen 24 MUI iv d for 2 w; BPen, 2.4 MUI im d for 3 w | 0.4/0.7 | 0.4/0.15 | 2 |
| 4 | 48/F | OU Blurred vision | OU choroidoretinitis; OU cataract | LN | Ceftriaxone 2 g iv d for 2 w; BPen, 2.4 MUI im d for 3 w | 0.05/0.4 | 1.0/0.8 | 22 |
| 5 | 52/M | OU Blurred vision, redness | OU papillioedema | HP | Ceftriaxone 2 g iv d for 2 w; BPen, 2.4 MUI im d for 3 w | 0.3/0.4 | 0.8/0.8 | 3 |
| 6 | 56/M | OS esotropia | OU CSCR; neurosyphilis; Argyll-Rovertson pupil | - | Ceftriaxone 2 g iv d for 2 w; BPen, 2.4 MUI im d for 3 w | 0.6/0.2 | UR | 1 |
| 7 | 55/M | OS blurred vision | OS papillioedema | - | BPen, 2.4 MUI im d for 3 w | 1.0/1.0 | 1.0/1.0 | 4 |
| 8 | 52/M | OU blurred vision | OU panuveitis; deafness | HIV+ | BPen, 2.4 MUI im d for 3 w | UR | UR | Transfers |
| 9 | 47/F | OS blurred vision | OS choroidoretinitis; OU DR | DM | Ceftriaxone 2 g iv d for 2 w; BPen, 2.4 MUI im d for 3 w | 0.8/0.5 | 0.8/0.6 | 3 |
| 10 | 44/F | OD blurred vision | OU choroidoretinitis | - | Ceftriaxone 2 g iv d for 2 w; BPen, 2.4 MUI im d for 3 w | 0.2/1.2 | 0.8/1.2 | 5 |
| 11 | 37/F | OD redness; blurred vision | OD anterior uveitis | HLA-B27 positive | BPen, 2.4 MUI im d for 3 w | 0.3/0.5 | 1.0/1.0 | 1 |
| 12 | 51/M | OD shadow float | OD choroidoretinitis | - | Ceftriaxone 2 g iv d for 2 w; BPen, 2.4 MUI im d for 3 w | 0.15/0.8 | 0.8/0.8 | 1 |
| 13 | 50/F | OS blurred vision | OS choroidoretinitis | - | Doxycycline 2.0 g P.Od for 30 d | 0.6/0.1 | 0.8/0.5 | 2 |
BCVA: best corrected visual acuity; R: right eye; L: left eye; BRVO: branch retinal vein occlusion; VH: vitreous hemorrhage; CSCR: chronic central serous chorioretinopathy; DR: diabetic retinopathy; LN: lupus nephritis; DM: diabetes mellitus; HP: hypertension; HIV: Human Immunodeficiency Virus; MS: multiple sclerosis; BPen: benzathine penicillin; Pen: aqueous penicillin G; AZM: azithromycin; UR: unrecorded.
Posterior segment involvement was found in 20 of 21 eyes (95.2%), while non-granulomatous anterior uveitis was present in one eye (4.8%). The most common manifestation was chorioretinitis (11 of 21 eyes, 52.4%; Table 1). FFA was performed in all patients, while ICGA was performed in 12 eyes of six patients (Table 2). The features found most frequently on FFA were disc hyperfluorescence (66.7%), retinal staining/capillary leakage (57.1%), and vessel staining (57.1%). “Leopard spots” (Figure 1) were found in five eyes (23.8%). A retinal non-perfusion area was observed in two eyes of two patients, whereas one patient with diabetes (patient 3) had branch retinal vein occlusion and one HIV-positive patient (patient 8) had panuveitis. The patterns most found frequently on ICGA were persistent dark spots (91.7%, Figure 1), fuzzy choroidal vessels (83.3%), and hot spots (75%).
Table 2.
The fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA) results
| N (%) | |
|---|---|
| Findings in FFA ( 21 eyes in 13 patients) | |
| Disc hyperfluorescence | 14 (66.7) |
| Retinal staining/capillary leakage | 12 (57.1) |
| Vascular staining | 12 (57.1) |
| Macular edema | 11 (52.4) |
| Leopard spots | 5 (23.8) |
| Retinal non-perfusion area | 2 (9.5) |
| Normal | 2 (9.5) |
| Findings in ICGA (12 eyes in 6 patients) | |
| Persistent dark dots | 11 (91.7) |
| Fuzzy choroidal vessels | 10 (83.3) |
| Hot spots | 9 (75) |
| Normal | 0 (0) |
Figure 1.
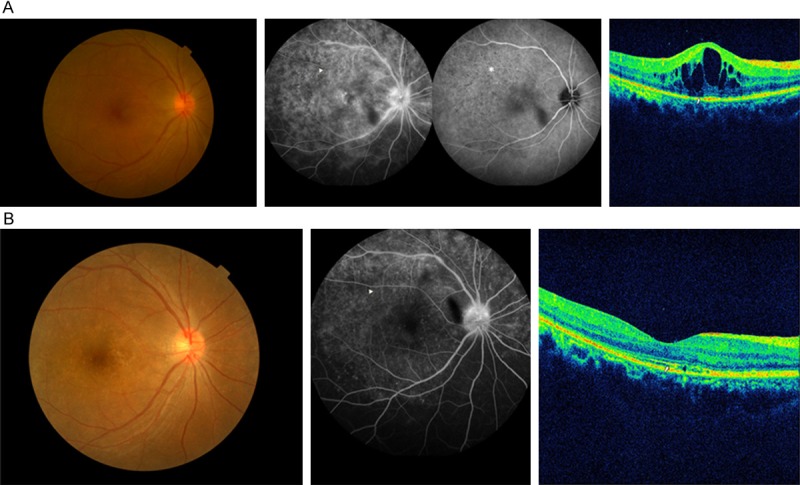
A. Disc hyperfluorescence, retinal staining, macular edema, and “leopard spots” (arrowhead) were present simultaneously on fundus fluorescein angiography (FFA) of patient 10. “Persistent dark dots” (asterisk) were present on indocyanine green angiography (ICGA). The inner segment/outer segment junction (IS/OS) loss (arrow) and cystoids macular edema (CME) were seen on spectral domain optical coherence tomography (SD-OCT). B. Six months after being treated with a neurosyphilis regimen, the FFA had improved significantly, and the “leopard spots” had disappeared (arrowhead). The IS/OS junction was mostly restored on SD-OCT (arrow), except for a slight disruption, and the CME was eliminated completely.
Macular SD-OCT (Table 3) was performed in 15 eyes of nine patients. The most common findings were inner segment/outer segment junction (IS/OS) loss in 13 eyes (86.7%) and subretinal fluid (SRF) and cystoids macular edema (CME) in two eyes each (13.3%, Figure 1). Hyper-reflective nodules on the retinal pigment epithelium (RPE, Figure 2), neuroepithelium layer edema, and elevated hyper-reflective subRPE were present in one eye each. The SD-OCT was normal in two eyes, including those of the patient with anterior uveitis.
Table 3.
Macular findings in spectral domain optical coherence tomography (SD-OCT) in 15 eyes of 9 patients
| N (%) | |
|---|---|
| IS/OS loss | 13 (86.7) |
| Subretinal fluid | 2 (13.3) |
| Cystoid macular edema | 2 (13.3) |
| Hyperreflective nodules on RPE | 1 (6.7) |
| Neuroepithelium layer edema | 1 (6.7) |
| Elevated hyperreflection of subRPE | 1 (6.7) |
| Normal | 2 (13.3) |
IS/OS: inner segment/outer segment junction; RPE: retinal pigment epithelium.
Figure 2.
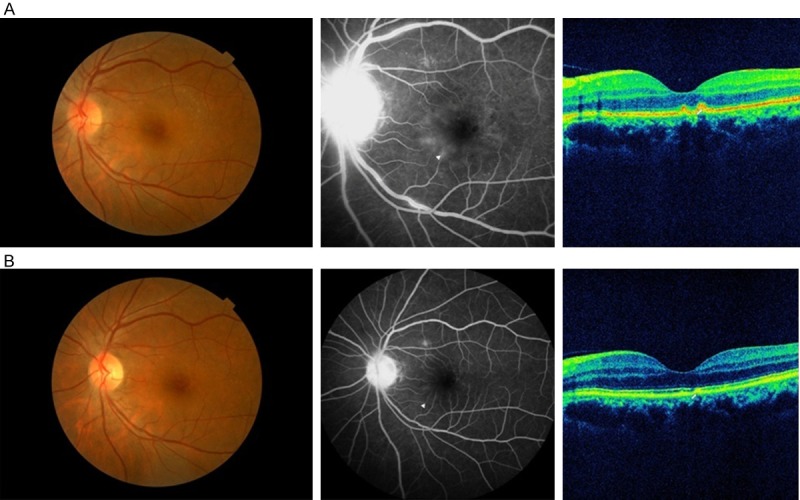
A. A yellow-white retinal lesion (placoid) of the macula was present in the fundus image of patient 13. Fluorescein angiography showed multiple leakages around the fovea (arrowhead) and disc hyperfluorescence. Spectral domain OCT (SD-OCT) showed nodules on the retinal pigment epithelium (RPE, arrow). B. Two months after being treated with oral doxycycline (200 mg daily) for 1 month, the fundus image was nearly normal. Fluorescein angiography showed that most of the leakage spots had vanished (arrowhead). The SD-OCT showed near normalization of the nodules on the RPE.
All of the patients presented with positive TPPA results (100%). A total of 13 of 14 patients presented with positive RPR results (92.9%, Table 4). The serum RPR ranged from 1:1 to 1:64. C-reactive protein (CRP) was tested in 13 patients, and an elevated CRP level (average 11.7 ± 4.0 (range 6.3-16.6 mg/L) was found in five patients (38.5%). Of the patients with an elevated CRP level, two had diabetes mellitus, one had lupus nephritis, and one had multiple sclerosis.
Table 4.
Auxiliary examination results of all subjects
| serum | CSF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| RPR | TPPA | HIV | CRP mg/L | RPR | TPPA | WBC Cells/μl | Protein mg/L | Glucose mmol/L | |
| 1 | 1:16 | + | - | 9.8 | - | - | 1 | 470 | 5.06 |
| 2 | 1:16 | + | - | 2.5 | 1:2 | + | 56 | 934.9 | 2.8 |
| 3 | 1:16 | + | - | 16.6 | - | - | 4 | 489.6 | 3.43 |
| 4 | 1:64 | + | - | 14.4 | - | - | 2 | 397.0 | 4.47 |
| 5 | 1:1 | + | - | 0.5 | - | - | 40 | 515 | 3.32 |
| 6 | 1:16 | + | - | 0.4 | 1:2 | + | 2 | 405 | 3.43 |
| 7 | 1:16 | + | - | 3.7 | - | + | 5 | 713.0 | 4.44 |
| 8 | 1:16 | + | + | NT | NT | NT | NT | NT | NT |
| 9 | - | + | - | 6.3 | - | + | 8 | 274 | 5.50 |
| 10 | 1:64 | + | - | 3.1 | 1:2 | + | 84 | 527.8 | 3.18 |
| 11 | 1:4 | + | - | 0.5 | - | + | 8 | 135 | 3.72 |
| 12 | 1:32 | + | - | 2.4 | - | - | 4 | 291 | 3.88 |
| 13 | 1:32 | + | - | 11.6 | NT | + | 120 | 896 | 3.34 |
RPR: Rapid Plasma Reagin; TPPA: Treponema pallidum particle agglutination; HIV: human immunodeficiency virus; CSF: cerebrospinal fluid; CRP: C reactive protein; WBC: white blood cells; BRVO: branch retinal vein occlusion; NT: no test.
A lumbar puncture was performed in 12 patients; it was refused in one patient and was not performed in the patient with HIV infection due to transfer to a special hospital. There were no complications associated with the lumbar puncture. Six patients (50%) had confirmed neurosyphilis; four patients (33.3%) had an elevated white blood cell (WBC) count or protein level and three patients had a reactive RPR (25%, Table 4). Among the patients with confirmed neurosyphilis, the average cerebrospinal fluid (CSF) protein level was 528.8 ± 327.1 mg/L and the average WBC count was 46.3 ± 48.7 cells/μL. Among those patients with unconfirmed neurosyphilis, the average CSF protein level was 479.3 ± 140.2 mg/L and the average WBC count was 9.3 ± 15.1 cells/μL.
Three patients were given weekly intramuscular injections of benzathine penicillin (2.4 mUI) for 3 weeks and nine patients were treated with a neurosyphilis regimen. Six patients (46.2%) received daily intravenous injections of ceftriaxone (2 g) for 2 weeks and weekly intramuscular injections of benzathine penicillin G (2.4 mUI) for 3 weeks. Two patients (15.4%) received daily intravenous injections of aqueous penicillin G (24 mUI) for 2 weeks and weekly intramuscular injections of benzathine penicillin G (2.4 mUI) for 3 weeks. Due to a penicillin allergy, one patient was treated with oral doxycycline (200 mg daily) for 30 days (Table 1). VA was recorded completely in 13 eyes of 9 patients. At the end of follow-up, VA was improved in eight eyes (61.5%), unchanged in four eyes (30.8%), and decreased in one eye, which exhibited simultaneous branch retinal vein occlusion and vitreous hemorrhaging.
Discussion
A global resurgence of ocular syphilis has been reported recently [17]. In Asia, a number of ocular syphilitic cases have been reported over the past decade. Yap et al. studied 18 eyes of 12 patients with ocular syphilis over 5 years and found obvious male dominance (91.7%). In this case series, 3 patients were co-infected with HIV. Anterior uveitis and panuveitis were the most common presentations. Only two patients (16.7%) presented with chorioretinitis [10]. Yang et al. studied 35 eyes of 19 patients in China with syphilitic uveitis over 7 years and reported that most of the patients were male, and that 4 were co-infected with HIV [18]. Vitreous opacities and diffuse retinitis were the major manifestations in that study. Our case series included nearly equal numbers of males and females (53.8% to 42.9%). No patients were homosexual, which is unlike the situation in developed countries such as the United States, where 67% of the syphilis occurs in younger men who have had sex with other men [19]. Furthermore, only one patient was co-infected with HIV, in contrast to most reports from developed countries [7,12]. The most common presenting ocular symptom in our study was blurred vision, consistent with an earlier report [11]. Chorioretinitis was the most common presentation.
There have been many published articles on the characteristics of ocular syphilis as determined by FFA and ICGA [15,16,20]. Those presentations seen on FFA included retinal/capillary leakage, vascular staining, disc hyperfluorescence, and macular edema. Gass et al. found that “Leopard spots” on FFA is a characteristic of acute syphilitic posterior placoid chorioretinitis [20]. Mora et al. reported two typical anomalies: late-phase scattered hyperfluorescent spots and persistent staining of retinal vessels [21]. Balaskas et al. further reported that dark dots on ICGA might indicate active inflammation and that hot spots might suggest a longer disease duration, while vascular staining on FFA seemed to be associated with severe ocular inflammation [16]. In our study, the most common finding on FFA was disc hyperfluorescence, in contrast to a report in which the most common finding was retinal staining, followed by disc hyperfluorescence and vascular staining [16]. “Leopard spots” , which was observed in five eyes, was a characteristic manifestation on FFA. Persistent dark dots on ICGA in our case series was the most common presentation, which was similar to the mentioned reports [16].
There are few descriptions of the SD-OCT characteristics of ocular syphilis. Recently, Pichi et al. described the SD-OCT appearance in patients with acute syphilitic posterior placoid chorioretinitis; 43.3% of the eyes presented with subretinal fluid, IS/OS disruption, and hyper-reflective thickening of the retinal pigment epithelium within 1-2 days of the initial presentation [22]. Lima et al. reported two cases of syphilitic outer retinopathy, in which one case presented with a loss of the IS/OS and the other presented with IS/OS disruption and an irregular retinal pigment epithelium [23]. Burkholder et al. described three cases of acute syphilitic posterior placoid chorioretinitis that demonstrated hyper-reflective nodularity, thickening of the retinal pigment epithelium, and disruption of the IS/OS [24]. In our case series, the most common presentation was loss of the IS/OS, followed by SRF and CME, which was consistent with the above-mentioned results. These presentations on SD-OCT were valuable, and might help us to judge whether the choroid was involved without performing ICGA. In addition, the prognosis of syphilitic posterior placoid chorioretinitis could be evaluated with SD-OCT.
Although there have been many reports on the changes of the CSF in patients with syphilis combined with HIV coinfection, few give the results for the CSF in ocular syphilis. Tucker et al. [8] reported that 57% of all patients who were co-infected with HIV had a positive CSF venereal disease research laboratory (VDRL) test result, while Li et al. [12] reported that 25% of all cases had a positive CSF VDRL test result. Amaratunge et al. stated that 83% of HIV-positive patients and 61% of HIV-negative patients had abnormal CSF findings, including confirmed neurosyphilis, a high WBC count, or an abnormally high CSF protein level [25]. In our case series, 83.3% of the patients had abnormal CSF findings and 50% of the patients had confirmed neurosyphilis. The CSF results were valuable for judging whether the cerebrum was involved. Although it is invasive, we recommend that a lumbar puncture be performed in patients with ocular syphilis.
The International Union against Sexually Transmitted Infections recommended that ocular syphilis should be treated with a neurosyphilis regimen [13]. Yap et al. also found that most patients with syphilitic uveitis resolved after being treated with an appropriate penicillin regimen [10]. Doris et al. reported the remission of six cases of syphilitic uveitis at the Manchester Uveitis Clinic following intramuscular treatment with procaine penicillin G and oral probenecid [17]. In our case series, most of the patients had an improved VA and ocular presentations after treating the syphilis. In addition, the ocular manifestations of two patients improved with benzathine penicillin therapy alone.
This study had a number of limitations. First, this was a retrospective case series. Second, the sample size was small; thus, selection bias may have been present. However, the measured results were correct and objective. In the future, large, multicenter, prospective studies need to be conducted in China. Ancillary tests, such as polymerase chain reaction (PCR) of aqueous humor and vitreous, which can help in making an accurate diagnosis immediately, need to be performed.
Conclusions
Our study reported the objective characteristics of ocular syphilis in Southeast China. The manifestations of ocular syphilis can mimic any eye disease. Chorioretinitis was the most common finding in this case series. “Leopard spots” was the characteristic manifestation on FFA. IS/OS loss was the most common finding in patients with posterior uveitis on SD-OCT. Lumbar puncture can contribute to the diagnosis of neurosyphilis. The treatment for ocular syphilis was effective in these patients.
Acknowledgements
This work was supported by the Department of Education of Zhejiang Province, China (Y201326908). Design and conduct of the study (J.S., L.G.F., Y.M.L); management, and interpretation of the data (J.S., L.G.F., Y.M.L); collection, analysis, and preparation (J.S., L.G.F., N.Zh), and approval of the manuscript (J.S., L.G.F., Y.M.L).
Disclosure of conflict of interest
None.
References
- 1.Brown AE, Sadler KE, Tomkins SE, McGarrigle CA, LaMontagne DS, Goldberg D, Tookey PA, Smyth B, Thomas D, Murphy G, Parry JV, Evans BG, Gill ON, Ncube F, Fenton KA. Recent trends in HIV and other STIs in the United Kingdom: data to the end of 2002. Sex Transm Infect. 2004;80:159–66. doi: 10.1136/sti.2004.009571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Primary and secondary syphilis-United States, 2003-2004. MMWR Morb Mortal Wkly Rep. 2006;55:269–73. [PubMed] [Google Scholar]
- 3.Hook EW 3rd, Peeling RW. Syphilis control: a continuing challenge. N Engl J Med. 2004;351:122–124. doi: 10.1056/NEJMp048126. [DOI] [PubMed] [Google Scholar]
- 4.Chen ZQ, Zhang GC, Gong XD, Lin C, Gao X, Liang GJ, Yue XL, Chen XS, Cohen MS. Syphilis in China: results of a national surveillance programme. Lancet. 2007;369:132–8. doi: 10.1016/S0140-6736(07)60074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205–16. doi: 10.1128/CMR.18.1.205-216.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiss S, Damico FM, Young LH. Ocular manifestations and treatment of syphilis. Semin Ophthalmol. 2005;20:161–7. doi: 10.1080/08820530500232092. [DOI] [PubMed] [Google Scholar]
- 7.Puech C, Gennai S, Pavese P, Pelloux I, Maurin M, Romanet JP, Chiquet C. Ocular manifestations of syphilis: recent cases over a 2.5-year period. Graefes Arch Clin Exp Ophthalmol. 2010;248:1623–9. doi: 10.1007/s00417-010-1481-z. [DOI] [PubMed] [Google Scholar]
- 8.Tucker JD, Li JZ, Robbins GK, Davis BT, Lobo AM, Kunkel J, Papaliodis GN, Durand ML, Felsenstein D. Ocular syphilis among HIV-infected patients: a systematic analysis of the literature. Sex Transm Infect. 2011;87:4–8. doi: 10.1136/sti.2010.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonollosa A, Giralt J, Pelegrín L, Sánchez-Dalmau B, Segura A, García-Arumí J, Adan A. Ocular syphilis-back again: understanding recent increases in the incidence of ocular syphilitic disease. Ocul Immunol Inflamm. 2009;17:207–12. doi: 10.1080/09273940902741709. [DOI] [PubMed] [Google Scholar]
- 10.Yap SC, Tan YL, Chio MT, Teoh SC. Syphilitic Uveitis in a Singaporean Population. Ocul Immunol Inflamm. 2014;22:9–14. doi: 10.3109/09273948.2013.829106. [DOI] [PubMed] [Google Scholar]
- 11.Anshu A, Cheng CL, Chee SP. Syphilitic uveitis: an Asian perspective. Br J Ophthalmol. 2008;92:594–7. doi: 10.1136/bjo.2007.133843. [DOI] [PubMed] [Google Scholar]
- 12.Li SY, Birnbaum AD, Tessler HH, Goldstein DA. Posterior syphilitic uveitis: clinical characteristics, co-infection with HIV, response to treatment. Jpn J Ophthalmol. 2011;55:486–94. doi: 10.1007/s10384-011-0053-z. [DOI] [PubMed] [Google Scholar]
- 13.French P, Gomberg M, Janier M, Schmidt B, van Voorst Vader P, Young H. IUSTI: 2008 European Guidelines on the Management of Syphilis. Int J STD AIDS. 2009;20:300–9. doi: 10.1258/ijsa.2008.008510. [DOI] [PubMed] [Google Scholar]
- 14.Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines. MMWR Recomm Rep. 2006;55:1–94. [PubMed] [Google Scholar]
- 15.Altan-Yaycioglu R, Akova YA, Akca S, Yilmaz G. Inflammation of the posterior uvea: findings on fundus fluorescein and indocyanine green angiography. Ocul Immunol Inflamm. 2006;14:171–9. doi: 10.1080/09273940600660524. [DOI] [PubMed] [Google Scholar]
- 16.Balaskas K, Sergentanis TN, Giulieri S, Guex-Crosier Y. Fluorescein and indocyanine-green angiography in ocular syphilis: an exploratory study. Graefes Arch Clin Exp Ophthalmol. 2012;250:721–30. doi: 10.1007/s00417-011-1893-4. [DOI] [PubMed] [Google Scholar]
- 17.Doris JP, Saha K, Jones NP, Sukthankar A. Ocular syphilis: the new epidemic. Eye. 2006;20:703–5. doi: 10.1038/sj.eye.6701954. [DOI] [PubMed] [Google Scholar]
- 18.Yang PZ, Zhang N, Li FZ, Chen Y, Kijlstra A. Ocular manifestations of syphilitic uveitis in Chinese Patients. Retina. 2012;32:1906–14. doi: 10.1097/IAE.0b013e3182509796. [DOI] [PubMed] [Google Scholar]
- 19.Ho EL, Lukehart SA. Syphilis: using modern approaches to understand an old disease. J Clin Invest. 2011;121:4584–4592. doi: 10.1172/JCI57173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97:1288–97. doi: 10.1016/s0161-6420(90)32418-1. [DOI] [PubMed] [Google Scholar]
- 21.Mora P, Borruat FX, Guex-Crosier Y. Indocyanine green angiography anomalies in ocular syphilis. Retina. 2005;25:171–81. doi: 10.1097/00006982-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Pichi F, Ciardella AP, Cunningham ET Jr, Morara M, Veronese C, Jumper JM, Albini TA, Sarraf D, McCannel C, Voleti V, Choudhry N, Bertelli E, Giuliari GP, Souied E, Amer R, Regine F, Ricci F, Neri P, Nucci P. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina. 2014;34:373–84. doi: 10.1097/IAE.0b013e3182993f11. [DOI] [PubMed] [Google Scholar]
- 23.Lima BR, Mandelcorn ED, Bakshi N, Nussenblatt RB, Sen HN. Syphilitic Outer Retinopathy. Ocul Immunol Inflamm. 2014;22:4–8. doi: 10.3109/09273948.2013.841960. [DOI] [PubMed] [Google Scholar]
- 24.Burkholder BM, Leung TG, Ostheimer TA, Butler NJ, Thorne JE, Dunn JP. Spectral domain optical coherence tomography findings in acute syphilitic posterior placoid chorioretinitis. J Ophthalmic Inflamm Infect. 2014;4:2. doi: 10.1186/1869-5760-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amaratunge BC, Camuglia JE, Hall AJ. Syphilitic uveitis: a review of clinical manifestations and treatment outcomes of syphilitic uveitis in human immunodeficiency. Clin Experiment Ophthalmol. 2010;38:68–74. doi: 10.1111/j.1442-9071.2010.02203.x. [DOI] [PubMed] [Google Scholar]


