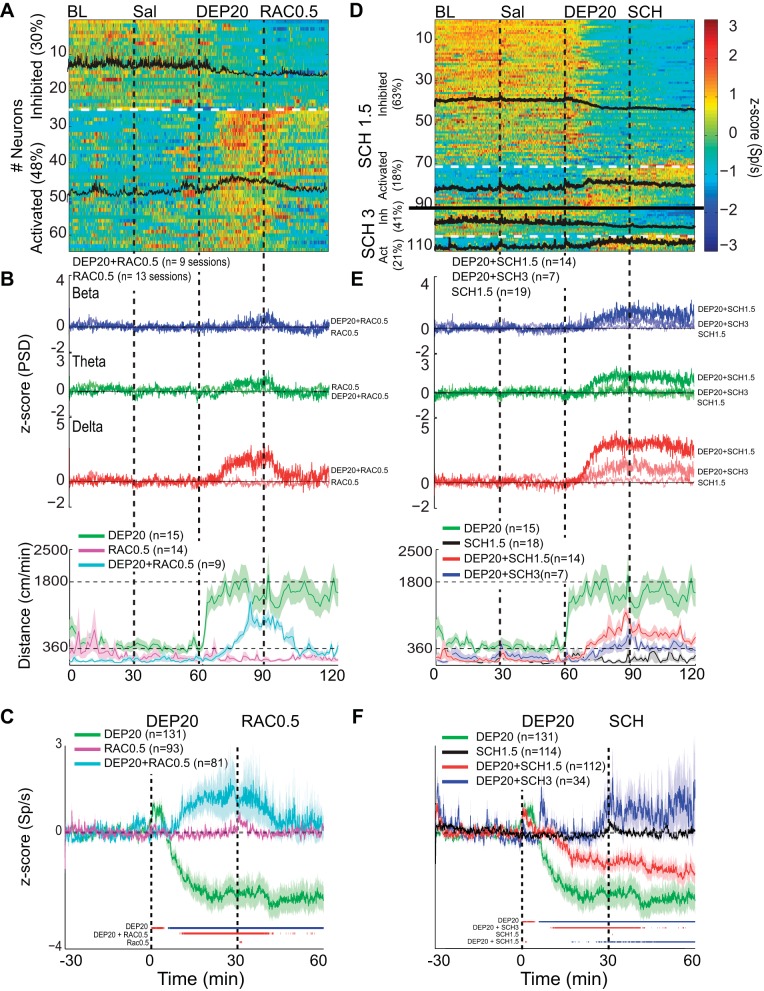
Fig. 10.
Intragastric dopamine D1R and D2R antagonists reverse the DEP20-induced inhibition/activation asymmetry in the rat's NAc shell. A: a plot of the activity of 64 (of 81) NAc shell neurons that were significantly inhibited (30%) or activated (48%) by DEP20 across 7 days of chronic treatment with RAC0.5. Each of the 4 epochs was 30 min long. Note that compared with DEP20 alone (only using neurons recorded over 7 days from Fig. 3B), the percentage of neurons inhibited during the DEP20 epoch in the presence of chronic treatments with RAC0.5 decreased and the percentage activated increased. The black traces are the mean activity of the 2 respective categories. B, top: the grand average of 9 experiments showing the normalized PSD at delta frequency from the same experiments shown in A over all days. Infusion of RAC0.5 greatly diminished the changes in all DEP20-induced oscillations. Note that infusion of RAC0.5 alone (thin traces) did not change LFP oscillations. Bottom, locomotion increases evoked by DEP20 were also decreased by RAC0.5 (cyan trace), whereas RAC0.5 alone did not increase locomotion (pink trace). C: a plot of the normalized global population responses of all neurons recorded (n = 131) with DEP20 alone (green; same data as in Fig. 3, aligned to DEP injection time = 0 min) vs. all 81 neurons recorded with DEP20 and chronic treatment with RAC0.5 (cyan trace over same treatment days, DEP20+RAC0.5). Note that after the infusion of RAC0.5, the population firing rate returned to near baseline levels. Finally, the infusion of RAC0.5 alone did not produce any inhibitory imbalance (pink trace). The colored lines at bottom indicate the bins (1-min resolution) with significant increases (red) or decreases (blue) of population activity relative to saline firing rates (time interval: −30 to 0 min). D: results showing repeated administration of two doses of D1R antagonist (SCH1.5 and SCH 3) infused after DEP20. The firing rate modulation of 91 out of 112 (and 21 out of 34) single neurons was recorded in the NAc shell during 30-min BL, Sal, DEP20+SCH1.5, and DEP20+SCH3 infusions, respectively. Under repeated administration SCH, DEP20 did not produce a robust spiking inhibition [SCH1.5 (63%) and SCH3 (41%) compared with DEP20 alone (63%, 82/131)]; rather, it increased its activation [SCH1.5 (18%) and SCH3 (21%) compared with DEP20 alone (11%, 14/131)]. E: a plot of the LFP PSD (top) and locomotor activity (bottom) induced by SCH1.5 (red trace), SCH3 (blue trace), and SCH1.5 alone (black trace). D1R antagonist attenuated in a dose-dependent manner the DEP20-induced delta oscillations and delayed the onset and diminished the magnitude of locomotion evoked by DEP20. Note all oscillations are positive. The infusion of SCH1.5 alone (thinnest lines) did not change LFP oscillations, with values around 0 z-score. F: the average firing rate of all neurons recorded under repeated treatment of DEP20 followed by SCH1.5 (red trace) and SCH3 (blue trace) and treatment of SCH1.5 alone (black trace). Note that SCH in a dose-dependent manner reversed the inhibition/activation imbalance induced by DEP20.