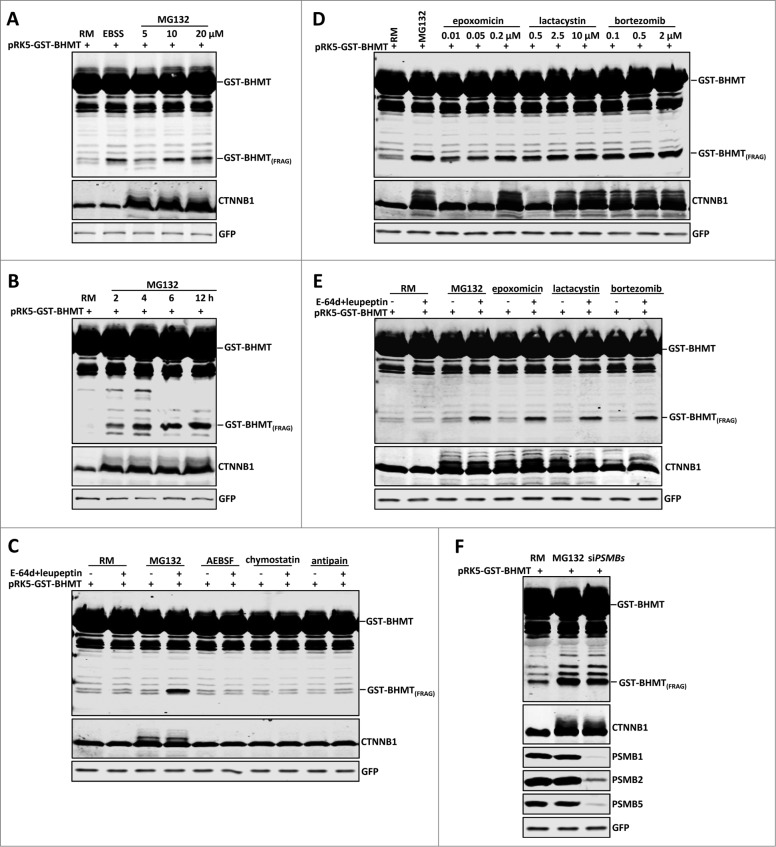
Figure 1 (See previous page).
Proteasome inhibition induces GST-BHMT fragmentation. HEK293T cells were transfected with 2 µg pRK5-GST-BHMT plasmids and incubated in the indicated medium followed by coimmunoprecipitation with anti-GST antibody and western blot analyses to detect the proteolytic processing of the GST-BHMT reporter. The prominent accumulation of CTNNB1, an endogenous proteasome substrate, confirmed the effective inactivation of the proteasome by MG132. GFP-MYC, whose expression is driven by the internal ribosome binding sites in the pRK5-GST-BHMT plasmid, was revealed by anti-MYC antibody and served as normalization control, as reported.20 (A) MG132 treatment induces GST-BHMT fragmentation. HEK293T cells were incubated in EBSS or in nutrient-rich medium (RM) containing MG132 at the indicated concentrations from 5 µM to 20 µM for 6 h, before being processed for the GST-BHMT assay. (B) Time-dependent analysis of GST-BHMT processing. After incubation in nutrient-rich medium with 10 µM MG132, transfected HEK293T cells were harvested at indicated time points from 2 h to 12 h. Notice the gradual accumulation of GST-BHMT(FRAG) product overtime, as revealed by western analysis with anti-GST antibody. (C) Inhibition of lysosomal proteases does not induce GST-BHMT fragmentation. HEK293T cells transfected with GST-BHMT reporter were treated with MG132 (10 µM) or with lysosome cysteine protease inhibitors AEBSF (2 mM), chymostatin (100 µM) or antipain (50 µg/ml), as indicated. Note the apparent accumulation of GST-BHMT(FRAG) only in the sample treated with both MG132 and lysosome protease inhibitors E-64d and leupeptin (lane 4). (D) Multiple proteasome inhibitors, including epoxomicin, lactacystin, and bortezomib, induce similar GST-BHMT processing as that by MG132. (E) The proteasome inhibition-induced accumulation of GST-BHMT(FRAG) requires the presence of lysosomal protease inhibitors E-64d and leupeptin. The transfected cells were treated in the presence or absence of E-64d and leupeptin together with different proteasome inhibitors, as indicated: MG132 (10 µM), epoxomicin (0.1 µM), lactacystin (2.5 µM), and bortezomib (0.5 µM). (F) Genetic interference of proteasomal function leads to similar BHMT fragmentation as that induced by MG132 (compare lanes 3 with 2). For sample in lane 3, cells were cotransfected with 5 nM of siRNAs against proteasome catalytic subunits PSMB1, PSMB2 and PSMB5, and their knockdown efficiency was verified by western blotting analysis, as indicated.