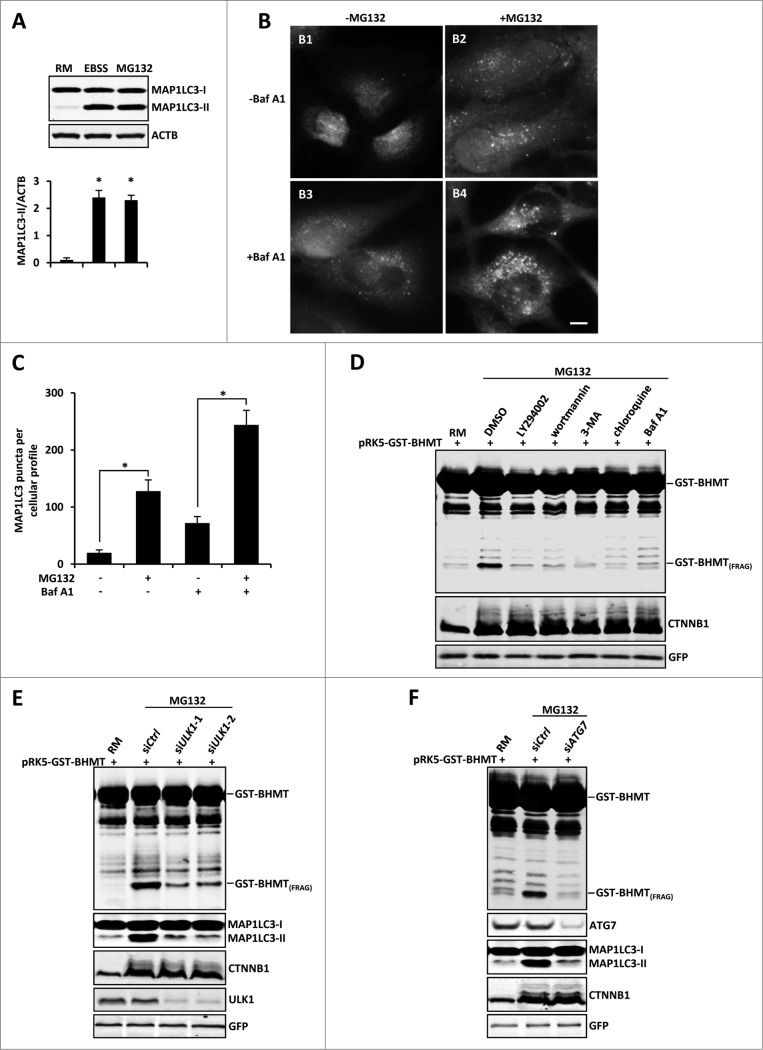
Figure 2 (See previous page).
Proteasome inhibition-induced GST-BHMT processing is autophagy-dependent. (A-C) Proteasome inhibition induces increased levels of MAP1LC3 lipidation and MAP1LC3-positive puncta formation. (A) For MAP1LC3 lipidation assay, HEK293T cells were treated with 10 µM MG132 in the presence of the lysosome inhibitor Baf A1 (100 nM). Both MAP1LC3-I and MAP1LC3-II were enriched by immunoprecipitation using antibody against MAP1LC3. Note that MG132 induced a similar level of MAP1LC3-II as by EBSS treatment. (B-C) For MAP1LC3-positive puncta formation assay, HeLa cells were treated with 10 µM MG132 in the absence or presence of lysosome inhibitor Baf A1 (100 nM), as indicated. Scale bar: 5 µm. (C) Quantitative analysis of cellular MAP1LC3-positive puncta profile. For each sample, MAP1LC3-positive puncta in about 200 cells were counted. The data was presented as the average number of MAP1LC3-positive puncta per cell. (D-F) Proteasome inhibition-induced BHMT processing is autophagy-dependent. (D) HEK293T cells were treated with MG132 (10 µM) together with DMSO control or the following pharmacological inhibitors of the autophagy pathway: LY294002 (100 µM), 3-methyladenine (3-MA; 10 mM), wortmannin (1 µM), chloroquine (100 µM), and Baf A1 (100 nM). Compared to the mock-treated sample using solvent DMSO, all other samples that were simultaneously treated with the indicated autophagy inhibitors showed reduced accumulations of GST-BHMT(FRAG). (E and F) GST-BHMT fragmentation was inhibited following the depletion of essential autophagy components. HEK293T cells were cotransfected with 10 nM siRNA against ULK1 (E) or ATG7 (F). Note that knockdown of either ULK1 (E) or ATG7 (F), but not treatment with control siRNA (siCtrl), significantly reduced the production of GST-BHMT(FRAG) and MAP1LC3 lipidation.