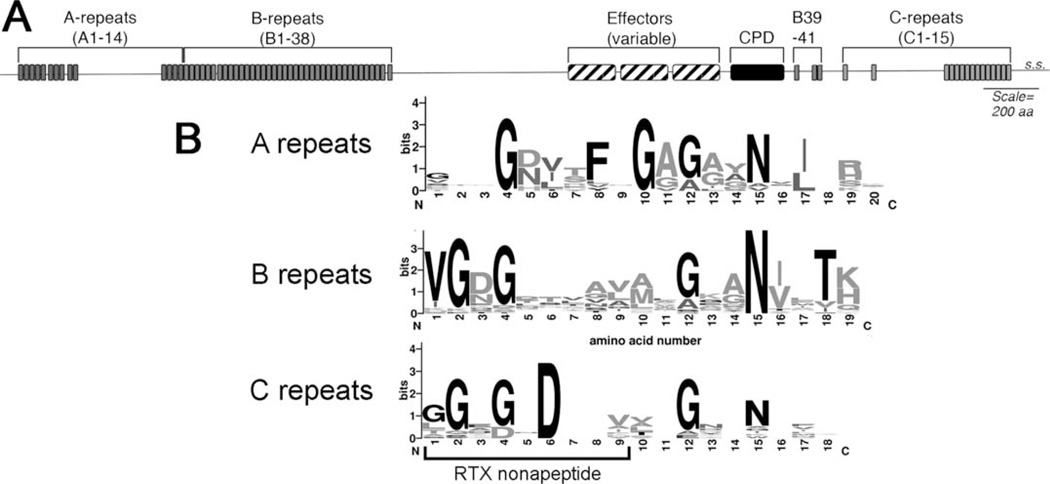
FIGURE 2.
(A) The general structure of a multifunctional-autoprocessing repeats-in-toxin (MARTX) toxin showing the number and position of various repeat sequences, auto-processing cysteine protease domain (CPD), and variable region containing the effector domains. The secretion signal (s.s.) is shown at the extreme C-terminus. (B) Graphical representation of the different repeat sequences generated by Weblogo 2.8.2 [(113); weblogo.berkeley.edu]. The sequences used were repeat sequences from Vibrio vulnificus CMCP6 identified based on the alignment of sequence to the repeat annotation of V. cholerae (1). The portion of the C-repeat that aligns to the calcium-binding beta roll nonapeptide repeat of other RTX family proteins is indicated. Note conservation of a G-7x–G-4x–N repeat in all of the repeats. doi:10.1128/microbiolspec.VE-0002-2014.f2