Figure 3.
Knocking Down and Knocking Out vgat Expression from Histaminergic Neurons in the TMN
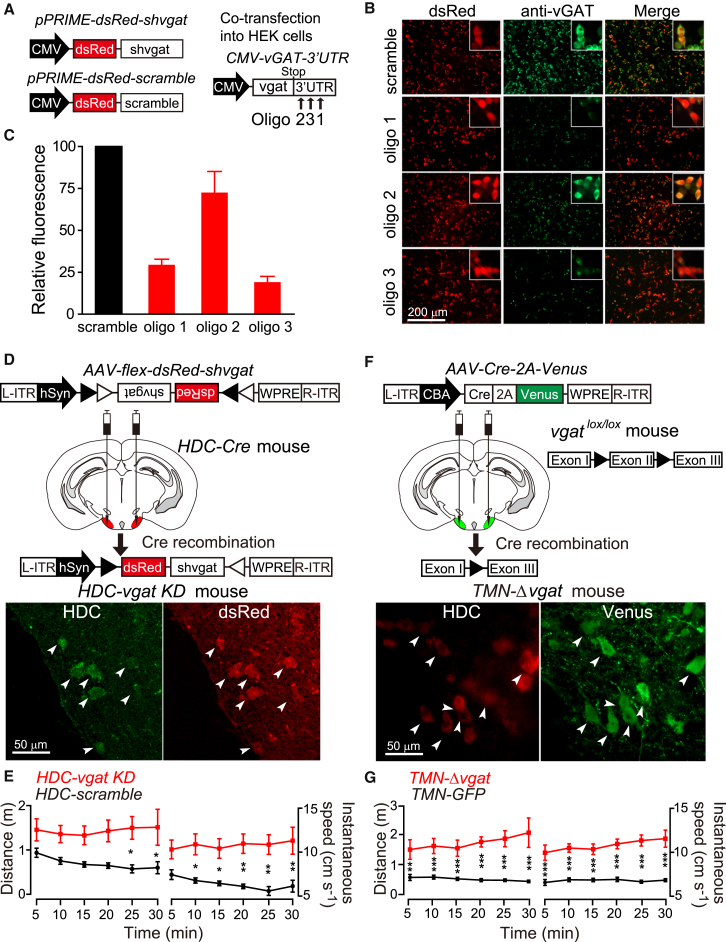
(A) Three hairpin (sh) oligonucleotides (sholigo), targeting the vgat transcript, were designed. Each sholigo was separately cloned into the pPRIME-dsRed vector; a scramble shRNA was also cloned into pPRIME-dsRed.
(B) To test the knockdown efficiency of the three shvgat oligonucleotides in vitro, CMV-dsRed-scramble or CMV-dsRed-shvgat (1, 2, or 3) were cotransfected with pCMV-vGAT-3′UTR (a cDNA containing the vgat open reading frame and 3′ untranslated region) into HEK293 cells. Thirty-six hours later, cells were fixed and stained with vGAT antisera. vGAT expression was best reduced with sholigo1 and sholigo3.
(C) Three independent transfections were performed, and relative vGAT immunofluorescence in HEK cells was quantified.
(D) AAV-flex-dsRed-shvgat (oligo3) was bilaterally injected into the TMN region of HDC-Cre mice. Cre recombination produced dsRed-shvgat expression in HDC-positive neurons, and the resulting mice were termed HDC-vgat KD mice. Arrowheads indicate examples of colabeled cells.
(E) HDC-vgat KD mice were more active than HDC-scramble (AAV-flex-dsRed-scramble-injected HDC-Cre) mice in an open field assay (∗p < 0.05; ∗∗p < 0.01).
(F) AAV-Cre-2A-Venus was bilaterally injected into the TMN region of vgatlox/lox mice to generate TMN-Δvgat mice. Sections from virus-injected (TMN-Δvgat) mice were costained with HDC and GFP (Venus) antisera. In the TMN region, GFP expression was in 77% ± 2% of HDC neurons. Arrowheads indicate examples of colabeled cells.
(G) TMN-Δvgat mice ran further than TMN-GFP (AAV-GFP-injected vgatlox/lox) mice in an open field assay (∗∗p < 0.01; ∗∗∗p < 0.001). During each running episode TMN-Δvgat mice also ran faster, as evidenced by instantaneous speed measurements (∗∗p < 0.01; ∗∗∗p < 0.001).