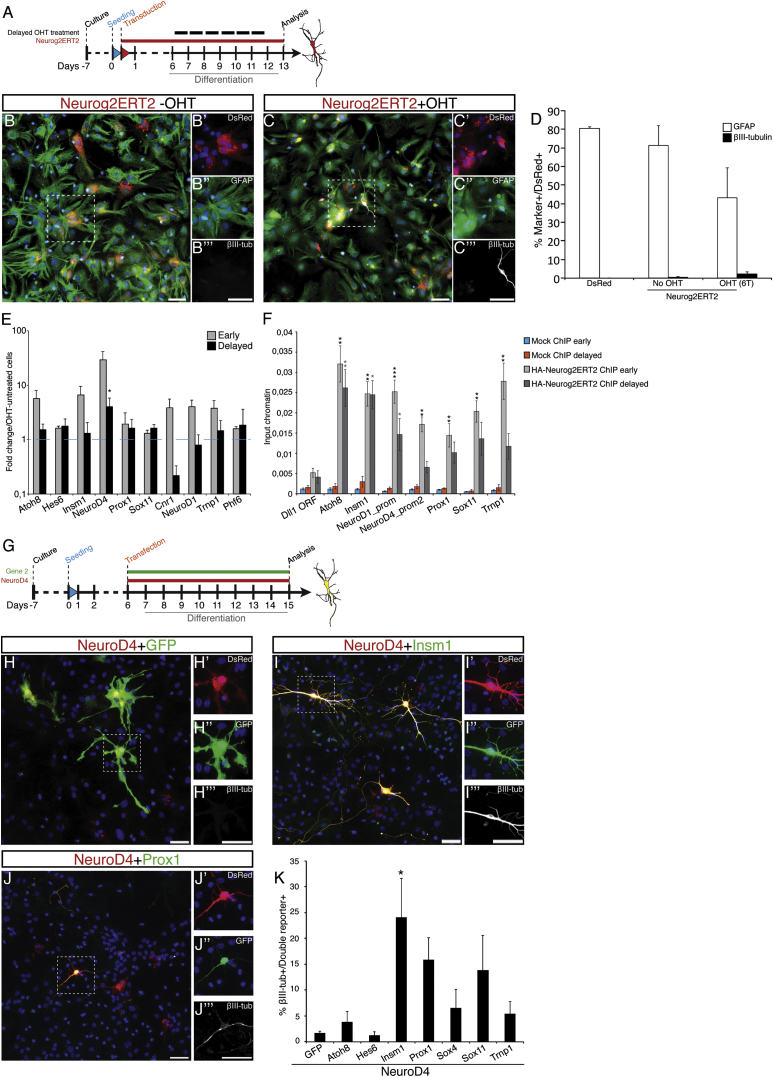
Figure 5.
Delayed Induction of Neurog2ERT2 Reveals a Block in Astrocyte Reprogramming
(A) Scheme of the experimental procedure.
(B and C) Micrographs of Neurog2ERT2-infected astrocytes (red) immunostained for GFAP (green) and βIII-tubulin (white), without (B) or with (C) OHT treatment starting at 6 days after being plated. Scale bars, 100 μm.
(D) Histogram depicting the proportion of GFAP+ or βIII-tubulin+ cells among infected cells upon delayed Neurog2ERT2 activation at 13 DPI. n = 4 independent experiments.
(E and F) Histograms of real-time qPCR (E) and HA-Neurog2ERT2 μChIP-PCR (F) of astrocyte cultures treated as indicated in the legend (early, early OHT treatment, gray bars in E from Figure 1A; and delayed, OHT treatment 6 days later). For (F) cells were exposed to OHT treatment for 24 hr. Percentages of input chromatin were quantified in duplicate from three independent biological samples (mean ± SEM). Significance was tested between samples and respective Dll1 ORF negative region by two-tailed unpaired t test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.0001).
(G) Scheme of the experiment.
(H–J) Micrographs of astrocytes transfected with the constructs indicated on top of the panels with a 5 day delay immune-stained for βIII-tubulin 8 days post transfection (DPT). Scale bars, 50 μm.
(K) Histogram depicting the proportion of βIII-tubulin+ cells at 8 DPT. Mean ± SEM; n = 4 independent experiments (∗p < 0.05).
See also Figure S5.