Abstract
Objective: Treatment of acne scarring is always a challenge. Microneedling therapy or percutaneous collagen induction is a new addition to the treatment modalities for such scars and has been reported to be simple and effective in atrophic acne scar treatment. The aim of this study is to evaluate the clinical effect and objectively quantify the histological changes of acne scarring in response to skin microneedling. Design: A prospective clinical study. Participants: Ten patients with different types of atrophic acne scars were subjected to three months of skin microneedling treatment (six sessions at two-week intervals). Measurements: Patients were photographed, and skin biopsies were obtained at baseline as well as one and three months from the start of treatment. Histometry for epidermal thickness and quantitative evaluation of total elastin; newly synthesized tropoelastin; collagen types I, III, and VII; and newly synthesized collagen were performed for all biopsies. Results: Compared to the baseline, patients’ evaluations revealed noticeable clinical improvement in atrophic post-acne scars in response to skin microneedling. There was a statistically significant increase (p<0.05) in the mean of collagen types I, III, and VII and newly synthesized collagen, while total elastin was significantly decreased (p<0.05) after the end of treatment. Conclusions: Multiple minimally invasive sessions of skin microneedling are an effective treatment for post-acne atrophic scars as it stimulates the repair processes with the advantage of being a relatively risk-free, in-office procedure with minimal patient recovery time.
Acne is a common condition seen in up to 80 percent of young people and in five percent of older adults. In some patients, the severe inflammatory response results in textural change in the superficial and deep dermis, leading to post-acne scars. Different terms and surgical techniques have been used to describe the types of acne and to improve the appearance of scarring.1,2
Different classification systems of varying complexities have been introduced to classify post-acne atrophic scars into many morphological types. However, the most basic and practical system classifies post-acne atrophic scars into the following three main types: 1) icepick, 2) rolling, and 3) boxcar scars. It is common for patients to have more than one type of scar.1,3
Multiple methods including topical preparations, dermabrasion, laser resurfacing, punch excision, punch elevation, subcutaneous incision, chemical peels, dermal grafting, and fillers, as well as fat transfer, implantation of autologous collagen and cultured and expanded autologous fibroblasts, focal treatment with trichloroacetic acid, and skin microneedling (automated or dermaroller) have been used to treat post-acne scars via enhancement of dermal extracellular matrix (ECM) proteins.2,4-7
It is thought that needles break collagen bundles in the superficial layer of the dermis that are responsible for scars with subsequent induction of more collagen immediately under the epidermis.2,8 It is currently used in the cosmetic art to treat several skin conditions, such as pigmentary disorders, wrinkles, post-acne atrophic scars, burn-related scars, and big pores and is also a part of percutaneous collagen induction (PCI) therapy.9,10 The technique involves puncturing the skin multiple times using needles, a tattoo gun, or roller.8,11,12
The roller is a drum-shaped device with very fine protruding stainless steel needles (0.25-3mm in length) that was designed by Fernandes12 to closely puncture the skin. When a needle penetrates into the skin, the injury causes localized damage and minor bleeding by rupturing fine blood vessels. A completely different picture emerges when hundreds of fine needle pricks are placed close to each other.13 Quite the opposite to the ablative lasers, the needles penetrate the epidermis, but do not remove it, thus needling procedure can be safely repeated if needed and is also applicable to areas where laser treatments or deep peelings cannot be done.14
Skin microneedling and all its therapeutic possibilities are now being widely discussed. A major concern with microneedling studies of post-acne scars treatment is that the clinical results are often dependent on the subjective estimate by physicians and/or volunteers. Meanwhile, studies concerning histopathological response after microneedling treatment are few.2 The aim of the present study is to evaluate the clinical effect and objectively quantify the histological changes in response to skin microneedling for treatment of post-acne atrophic scarring.
PATIENTS AND METHODS
Subjects. The present study was conducted on 10 patients (five males and five females) with Fitzpatrick skin type III to IV complaining of different types of post-acne atrophic scars. The study was approved by the postgraduate and research committee of Al-Minya University, Al-Minya, Egypt. Patients, ranging in age from 19 to 32 years, with an average age of 26±5.1, were recruited from the dermatology outpatient clinic of Al-Minya University Hospital. Treatment and study details were fully explained to every patient before obtaining informed consent for treatment, study participation, and taking photographs and skin biopsies.
Punch biopsies (3mm) were obtained from the treatment area of patients at baseline, after one month (during treatment; 2 weeks after 2 sessions) and three months after the start of treatment (post-treatment; 2 weeks after 6 sessions). Skin samples were fixed in 10% buffered formalin, embedded in paraffin, and sectioned into 5um-thick sections.
Treatment protocol. Patients were subjected to three months of treatment (6 sessions at 2-week intervals). Erythema, edema, hypo- or hyperpigmentation were monitored during each visit.
The face was thoroughly cleaned with saline and povidone iodine. A topical anesthetic cream (lidocaine 2.5% and prilocaine 2.5%) was applied to the treatment area as a thick coating and left for 60 minutes under occlusion and then the cream was gently removed. Dermaroller with 1.5mm-long needles (Directive MDD 93/42 EEU, Dermaroller Deutschland S.a.r.L, Germany, 192 needles in 8 rows, needle diameter at penetration point: 0.25mm, width and diameter of the roller head: 20mm) was used. The treatment area was rolled in eight directions (vertical, up, down, horizontal, to the right, to the left, and in both diagonal directions) applying minimal pressure. The microneedles penetrate through the epidermis, which is only punctured and heals rapidly. Both the stratum corneum and the epidermis remain intact except for the minute holes. The endpoint for any treatment session was the presence of uniform bleeding points over the scarred area. In patients with deep-seated scarring, the skin was stretched in a perpendicular direction to the dermaroller movement so that the base of the scars could also be reached. After treatment, when bleeding stopped, the formed serous ooze is removed from the surface of the skin using sterile saline solution.
All patients were instructed to apply topical antibiotic “fusidic acid” for 24 hours, to guard against secondary infection, then moisturizing cream until the erythema resolves. Also, sun exposure was avoided by using sunscreens with a sun protection factor (SPF) value of 30 or more during the day.
Histology and immunostaining. Histopathological studies were performed using standard hematoxylin and eosin (H & E), Verhoeff-van Gieson (elastic fibers) (HT25A; Sigma Aldrich, St. Louis, Missouri), and picrosirius red staining (Direct Red 80, Sigma Aldrich) for newly synthesized collagen. When tissues are stained with picrosirius red and viewed under polarized microscope, large collagen fibers stain red while the thinner ones, which represent the newly synthesized fibers, are stained yellow to orange.15,16 The immunoperoxidase (IP) technique was used for evaluation of collagen types I and III, as well as total elastin.17-19 Briefly, tissue slides were heated at 60°C for 30 to 60 minutes and then deparaffinized and antigens retrieved in 0.1M sodium citrate (pH 6.0) for five minutes by the microwave method. Nonspecific sites were blocked and tissues were incubated with antibodies to collagen type I (1:400; sc-59772; Santa Cruz Biotechnology), collagen type III (1:600; ab6310; Abeam, Cambridge, Massachusetts), and total elastin (1:300; E4013; Sigma Aldrich). Samples were then incubated with biotinylated secondary antibody (1:200; PK-6102; Vector Labs, Burlingame, California), ABC reagent (Vectastain Elite ABC Peroxidase Kits Mouse; PK-6102; Vector Labs), and stained with DAB Chromogen Substrate Kit (K3468; Dako, CA, USA). All slides were counterstained with hematoxylin (7211; Thermo Scientific) and mounted for viewing.
Immunofluorescence (IF) was used to detect type VII collagen and tropoelastin. After deparaffinization and antigen retrieval, tissue slides were incubated with antibodies to type VII collagen (1:600; sc-33710; Santa Cruz Biotechnology) and to tropoelastin (1:400; Elastin Products, Owensville, Missouri), secondary antibody and 4’,6-Diamidino-2-phenylindole dihydrochloride (DAPI) (1:1000; D8417; Sigma Aldrich) for nuclear staining.
Quantitative evaluation of picrosirius red staining and immunostained tissues was carried out using computer-based software (Analysis® Five, Olympus Soft Imaging Solutions GmbH, Mtinster, Germany); a representative square area of lxlcm was used to measure luminosity for fluorescent stained sections, while another 2.5x2.5cm square was used to measure the color density for immunoperoxidase staining. All values were normalized to the baseline.
All histometric evaluations were carried out using computer-based software; the epidermal thickness was measured between the top (from the upper part of granular cell layer) to the bottom (dermoepidermal junction) of the rete ridges. Five measurements were calculated for each tissue using a computerized software analyzer.
Statistical analysis. Quantitative evaluations of histological measurements were analyzed using the Software Package for Statistical Science (SPSS) (SPSS for Windows; Version 16, SPSS Inc.; Chicago, Illinois). Statistical analysis was performed using One-way ANOVA, Wilcoxon-matched pairs signed ranks, and Chi-square tests.
Data was expressed as mean value ± standard deviation (SD). Statistical significance was defined as p≤0.05.
RESULTS
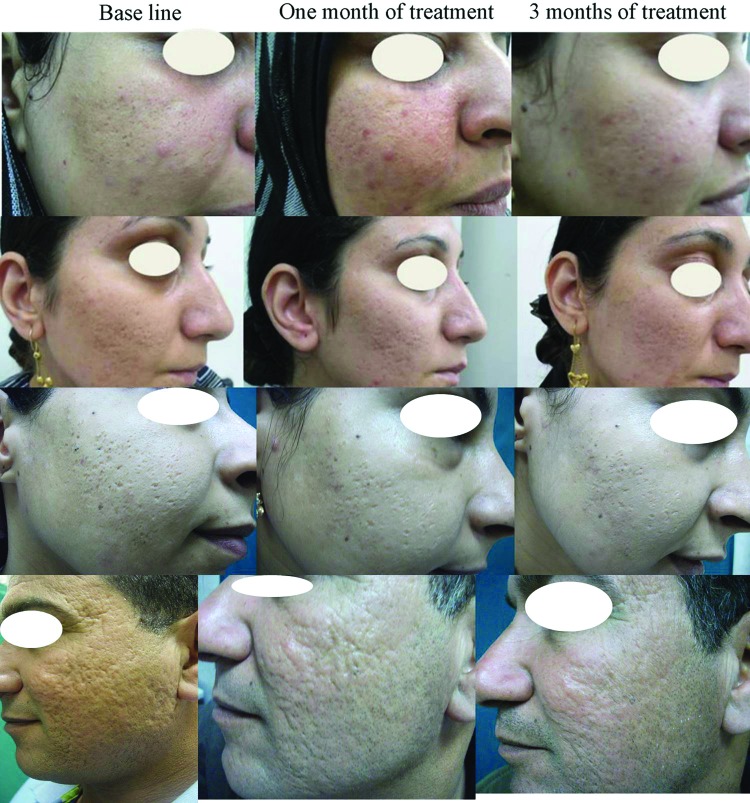
Clinical assessment. The authors have analyzed the efficacy of microneedling on different types of post-acne atrophic scars and skin texture together with overall patient satisfaction. The clinical improvement and changes were estimated and evaluated by the patients, two dermatologists, and two independent observers before treatment and at one and three months after the start of the treatment, based on a five-point scale (none=0%, mild=l-25%, moderate=26-50%, good=51-75%, and very good=76-100%). All patients completed the study and showed clinical improvement of post-acne atrophic scars appearance and skin texture. A noticeable enhancement in skin appearance, post-acne scars, and patient satisfaction was observed in response to skin microneedling treatment when compared to baseline (Figure 1).
Figure 1.
Representative photographs of patients with post-acne scars in response to skin microneedling therapy showing clinically noticeable improvement after one month of treatment, which continued to improve after three months of treatment when compared to baseline.
On correlating the response with the type of post-acne atrophic scarring, the present study showed good to very good 3 month s ot treatment response in rolling and boxcar scars ^’ while icepick scars showed only moderate improvement. However, many deep atrophic scars showed a poor response to treatment.
All patients reported facial edema and slight pain after the procedure, which resolved 24 hours later. By the second day, there was only mild erythema that resolved completely 1 to 2 days postoperative. Patients were able to return to normal daily activity two days after the procedure.
Pearson Chi-square test for statistical significance was used to analyze data obtained from the structured questionnaire. Compared to baseline, after one month of treatment, patients showed mild improvement (15-20%) in post-acne scars appearance (p=0.02) and skin texture (20-25%) (p= 0.02) and moderate overall satisfaction (30-40%) (p=0.01). Three months after starting treatment, significant differences were noticed among patients as they showed 51 to 60 percent (good) improvements in the appearance of post-acne scars (p=0.01), 40 to 50 percent (moderate) improvement in skin texture (p=0.01), and the patient satisfaction increased to 80 to 85 percent (very good) (p=0.001). Regarding doctor and observer assessment rates, data obtained were comparable to patient evaluation rates (Table 1).
TABLE 1.
Percent rate of clinical improvement relative to base line, following skin microneedling treatment of post acne atrophic scars
| PERCENT IMPROVEMENT (%) (n=10) | |||
|---|---|---|---|
| Post-acne scars appearance | Skin texture | Overall satisfaction | |
| After one month of treatment (after 2 sessions) | 15-20 mild | 20-225 mild | 30-40 * moderate |
| After three months post-treatment (after 6 sessions) | 51-60 * good | 40-50 * moderate | 80-85 * very good |
p <0.05
Histometric evaluation of the epidermis. Compared to the baseline, histometric evaluation of H&E stained sections revealed a significant increase of epidermal thickness from 63±4.9um to 72±7.1um after one month of treatment and to 80.2±3.8µm three months after starting treatment (p=0.01 and 0.004, respectively) (Table 2 and Figure 2).
TABLE 2.
Quantitative analysis of epidermal thickness and extracellular matrix proteins before and after microneedling treatment for post-acne atrophic scars
| (n=10) | (MEAN ± SD) | P-VALUE | ||||
|---|---|---|---|---|---|---|
| BASELINE | AFTER 1 MONTH OF TREATMENT (AFTER 2 SESSIONS) | AFTER 3 MONTHS POST-TREATMENT (AFTER 6 SESSIONS) | BASELINE VS. 1 MONTH OF TREATMENT | 1 MONTH OF TREATMENT VS. 3 MONTHS AFTER STARTING TREATMENT | BASELINE VS. 3 MONTHS AFTER STARTING TREATMENT | |
| Epidermal thickness (µm) | 63 ±4.9 | 72 ±7.1 | 80.2 ±3.8 | 0.01* | 0.02* | 0.004* |
| Collagen I (%) | 67.1 ±4.2 | 70.4 ± 5.4 | 78.2 ±6.8 | 0.175 | 0.03* | 0.01* |
| Collagen III (%) | 61.4 ±3.6 | 65.5 ±6.1 | 74.3 ± 7.4 | 0.07 | 0.02* | 0.01* |
| Collagen VII (%) | 15.2 ± 2.1 | 16.4 ±1.7 | 21.3 ±1.2 | 0.431 | 0.04* | 0.03* |
| Newly synthesized collagen (%) | 14.5 ±5.8 | 15.8 ±5.3 | 19.5 ±3.2 | 0.379 | 0.03* | 0.02* |
| Total elastin (%) | 51.3 ±6.7 | 50.1 ±4.7 | 46.9 ±4.3 | 0.492 | 0.05* | 0.04* |
| Tropoelastin (%) | 13.6 ±3.5 | 14.8 ±2.9 | 18.2 ±2.1 | 0.412 | 0.03* | 0.02* |
p<0.05
Figure 2.
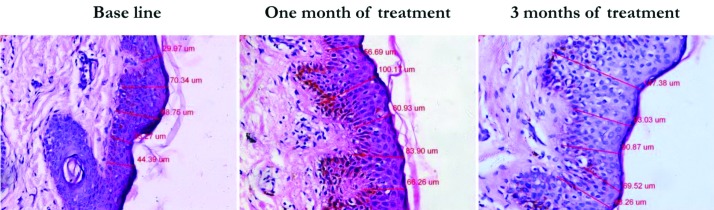
Histometric measurement showing enhanced epidermal thickness after one month and three months of microneedling treatment compared to baseline (H&E; x400)
Dermal changes. Quantitative assessment of the percent area of dermis occupied by immunohistochemically detectable collagen was done to evaluate the effect of microneedling treatment for post-acne atrophic scars on collagen types I and III, and then values were compared to the baseline for statistical significance. Collagen type I studies showed a statistically nonsignificant (p=0.175) increase in level from 67.1 ±4.2 percent before treatment to 70.4±5.4 percent after one month of starting skin microneedling treatment; this was followed by a statistically significant (p=0.01) increase to 78.2±6.8 percent three months post-treatment (Table 2 and Figure 3).
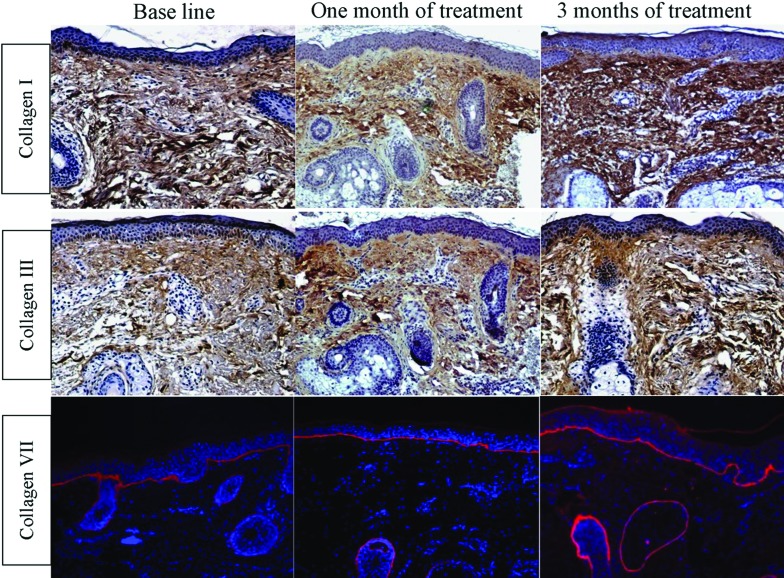
Figure 3.
Increase in the level of collagen in response to skin microneedling therapy for post-acne atrophic scars. Immunohistochemical staining of skin tissues at baseline, one month after treatment, and three months after starting microneedling treatment for collagen type I (1st row), collagen type III (2nd row), and collagen type VII (3rd row), compared to baseline (IP “1st and 2nd rows” and IF “3rd row” staining; x200)
Collagen type III showed a slight, but nonsignificant increase (p=0.07) from 61.4±3.6 percent at the baseline to 65.5±6.1 percent after one month of treatment, but the level of collagen type III expression increased significantly (p=0.01) three months after starting treatment (74.3±7.4 %) when compared to baseline (Table 2 and Figure 3).
Both fibroblasts and keratinocytes share in the synthesis of type VII collagen, the main component of anchoring fibrils, which partly mediates the dermoepidermal adherence in human skin.20 Type VII collagen expression was statistically insignificantly (p=0.431) increased from 15.2±2.1 percent before treatment to 16.4±1.7 percent after one month of skin microneedling treatment, meanwhile it was statistically significantly increased (p= 0.02) to 21.3±1.2 percent at three months after starting treatment when compared to baseline (Table 2 and Figure 3).
On evaluating newly formed collagen, skin microneedling therapy showed a favorable effect on enhancing collagen formation, as picrosirius red staining revealed a slight, but statistically nonsignificant (p= 0.379), increase from 14.5±5.8 percent before treatment to 15.8±5.3 percent after one month of treatment, yet this was followed by a statistically significant (p=0.02) increase to 19.5±3.2 percent three months after starting treatment when compared to baseline (Table 2 and Figure 4).
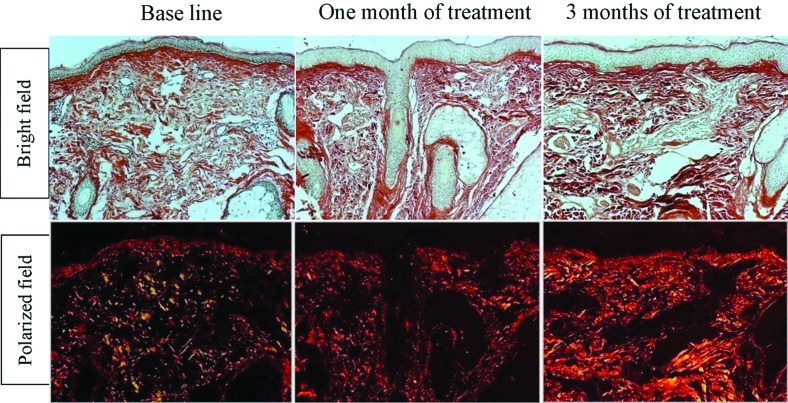
Figure 4.
Representative examples of skin biopsies stained with picrosirius red viewed under bright field microscope (top panels) and polarized field (bottom panels) showing increase in newly synthesized collagen level in response to skin microneedling treatment. Bright field captures total collagen content (in red). Polarized light shows a yellow to orange birefringence for newly synthesized collagen (Picrosirius red; x200)
Elastin level decreased from 51.3±6.7 percent at baseline to 50.1±4.7 percent after one month of treatment (p= 0.492), this was followed by continued decrease in elastin level to 46.9±4.3 percent at three months after starting treatment, which was statistically significant (p=0.04) when compared to baseline (Table 2 and Figure 5).
Figure 5.
Elastic tissue after skin microneedling treatment. Skin samples at baseline, after one month and three months post-treatment were immunostained for total elastin (top row) showing a decrease in total elastin level. Immunofluorescence staining of tropoelastin (bottom row) shows increased deposition of newly synthesized tropoelastin in the dermis (IP “1st row” and IF “2nd row” staining; x200)
The biosynthesis rate of normal elastin was evaluated by quantifying for newly synthesized tropoelastin, the precursor of elastin.17,21 Quantitative evaluation of tropoelastin showed minor, but statistically nonsignificant (p= 0.412), increase in the mean level from 13.6±3.5 percent to 14.8±2.9 percent after one month of skin microneedling therapy. However, compared to baseline, the content of tropoelastin showed statistically significant (p= 0.03) increase in level to 18.2±2.1 percent at three months after starting treatment (Table 2 and Figure 5).
DISCUSSION
Minimally invasive procedures are new modalities used for skin rejuvenation, tightening, and scar remodeling. They enhance dermal ECM proteins without ablation of the epidermis, therefore limiting side effects and minimizing downtime.7,22 Many studies concerning the efficacy of minimally invasive modalities have been published, yet most of them were based on subjective evaluation of the results.23-25 Simple and definitive treatments for acne scarring are few; meanwhile, microneedling therapy is a recent addition to the treatment armamentarium for managing post-acne atrophic scars.26 The authors have used dermaroller simple skin needling device to objectively evaluate the efficacy of skin microneedling therapy on post-acne atrophic scars, both on the clinical and histological levels.
Different studies reported that skin microneedling induces normal wound healing,2,3,10,27 developing in three consecutive stages. Stage 1, the inflammation stage, starts shortly after the injury in which platelets release chemotactic factors causing invasion of other platelets, neutrophils, and fibroblasts. During the proliferation stage (stage 2), after neutrophils are replaced, monocytes change into macrophages and release numerous growth factors including platelet-derived growth factor, fibroblast growth factor, and transforming growth factors alpha and beta, which stimulate the migration and proliferation of fibroblasts. Keratinocytes then start to reestablish the basement membrane by enhancing the production of laminin and collagen types IV and VII. Stage 3, the remodeling stage, continues for months after the injury and is mainly achieved by the fibroblasts; collagen is formed in the upper dermis over a period of a year or longer.2,28
Collagen III is the main type of collagen formed in the early wound healing phase. It is gradually replaced by collagen I over a period of a year or more resulting in continued tissue remodeling for months after the injury. Collagenases and matrix proteinases are involved in the gradual conversion of collagen III into collagen I, which remains in the area for 5 to 7 years.14
In the present study, clinical evaluation of patients showed statistically significant (p<0.05) overall improvement of post-acne atrophic scars, skin texture, and patient satisfaction in response to skin microneedling treatment when compared to baseline. Patients showed mild clinical improvement in post-acne scars and skin texture after one month of treatment (2 sessions) and reported moderate satisfaction. Meanwhile, clinical assessment at three months post-treatment (after 6 sessions) showed good enhancement of scar appearance and moderate improvement in skin texture, meanwhile patient satisfaction was very good when compared to the baseline (p<0.05) (Table 1). Very good response was seen in rolling and boxcar scars, while moderate response was seen in icepick scars.
After PCI, the patients reported transient erythema and edema, which resolved two days later. The result of the present work is consistent with previously published studies using skin microneedling technique for treatment of post-acne scars. These studies evaluated the efficacy of skin microneedling by using the dermaroller in the management of atrophic acne scars and showed improvement of acne scars in all patients without any side effects apart from redness and swelling, which disappeared within 2 to 3 days.2,27,29
To the best of the authors’ knowledge, no previous work concerning quantitative changes of collagen (newly formed, types I, III, and VII), elastin, and tropoelastin in response to skin microneedling therapy for post-acne atrophic scars were reported. The results of the present study improved the subjective estimate in the context of objective means by quantitatively evaluating the role of skin microneedling treatment for post-acne scars on ECM remodeling.
Quantitative evaluation of histological changes showed a statistically significant (p<0.05) increase of the epidermal thickness with enhanced formation of dermal papillae. Wounds created by microneedling to the papillary dermis initiate the normal process of wound healing, resulting in the synthesis of collagen types III and I. Assessment of ECM proteins showed statistically significant (p<0.05) enhancement of collagen and elastin deposition and formation in response to skin microneedling therapy. There was slight, but statistically nonsignificant (p>0.05) increase in collagen (types I, III, VII and newly synthesized) and tropoelastin, which was accompanied by a decrease in total elastin level after one month of treatment. These changes continued to show statistically significant (p<0.05) enhancement three months after starting treatment. The results of the present study showed that collagen type III started to increase first followed by subsequent increase in collagen type I content (Table 2). With the conversion of collagen type III into collagen type I,14 tightening occurs gradually with subsequent improvement of scar appearance.
The authors’ results were in agreement with different studies that used H&E staining to evaluate the effect of microneedling on post-acne atrophic scars treatment.6,14,27 Aust et al6 showed a 40-percent increase of thickening of the epidermis and normal rete ridges at one year postoperatively, which was accompanied by a significant increase in collagen deposition at six months postoperatively.6 Fernandes and Signorini14 showed that the skin became thicker with much greater collagen deposition and significantly more elastic fibers.
The present study also showed that multiple sessions (6 sessions) of minimally invasive microneedling (using 1.5mm dermaroller) initialize collagen synthesis and do not interfere with the normal lifestyle of the patient. On the other hand, other investigators14 used a 3mm dermaroller to produce better results, but with longer downtime and more liability for side effects and complications.
CONCLUSION
Skin microneedling is a simple, inexpensive office modality of treatment for the management of post-acne atrophic scars. It has favorable advantages as the epidermis remains intact, decreasing most of the risks and negative side effects of other invasive modalities for treatment of post-acne scars. The findings of the present study propose that the full result may take months to be achieved, as the deposition of new collagen takes place gradually, suggesting that continued skin microneedling treatment is required to achieve the desired clinical and histological improvement. However, further in-depth, long-term studies with a larger number of patients, using different size microneedling devices, are needed to evaluate the maximum duration of treatment required for each of them to achieve optimal effect with minimal downtime, side effects, and complications.
Footnotes
DISCLOSURE:The authors report no relevant conflicts of interest.
REFERENCES
- 1.Jacob CI, Dover JS, Kaminer MS. Acne scarring: a classification system and review of treatment options. J Am Acad Dermatol. 2001;45:109–117. doi: 10.1067/mjd.2001.113451. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrocini G, Fardella N, Monfrecola A, et al. Acne scarring treatment using skin needling. Clin Exp Dermatol. 2009;34:874–879. doi: 10.1111/j.1365-2230.2009.03291.x. [DOI] [PubMed] [Google Scholar]
- 3.Fife D. Practical evaluation and management of atrophic acne scars: tips for the general dermatologist. J Clin Aesthet Dermatol. 2011;4:50–57. [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes D. Minimally invasive percutaneous collagen induction. Oral Maxillofac Surg Clin North Am. 2005;17:51–63. doi: 10.1016/j.coms.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Mezzana P, Valeriani M. Rejuvenation of the aging faces using fractional photothermolysis and intense pulsed light: a new technique. Acta Chir Plast. 2007;29:47–50. [PubMed] [Google Scholar]
- 6.Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles and skin laxity. Plast Reconstr Surg. 2008;121:1421–1429. doi: 10.1097/01.prs.0000304612.72899.02. [DOI] [PubMed] [Google Scholar]
- 7.Habbema L, Verhagen R, Van Hal R, et al. Minimally invasive non-thermal laser technology using laser-induced optical breakdown for skin rejuvenation. J Biophotonics. 2012;5:194–199. doi: 10.1002/jbio.201100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orentreich DS, Orentreich N. Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatol Surg. 1995;21:543–549. doi: 10.1111/j.1524-4725.1995.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 9.Birchall JC, Clemo R, Anstey A, et al. Microneedles in clinical practice-an exploratory study into the opinions of healthcare professionals and the public. Pharm Res. 2011;28:95–106. doi: 10.1007/s11095-010-0101-2. [DOI] [PubMed] [Google Scholar]
- 10.El-Domyati M, Medhat W. Minimally invasive facial rejuvenation: current concepts and future expectations. Expert Rev Dermatol. 2013;8:565–580. [Google Scholar]
- 11.Camirand A, Doucet J. Needle dermabrasion. Aesth Plast Surg. 1997;21:48–51. doi: 10.1007/s002669900081. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes D. Percutaneous collagen induction: an alternative to laser resurfacing. Aesthet Surg J. 2002;22:307–309. doi: 10.1067/maj.2002.126195. [DOI] [PubMed] [Google Scholar]
- 13.Doddaballapur S. Microneedling with dermaroller. J Gutan Aesthet Surg. 2009;2:110–111. doi: 10.4103/0974-2077.58529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008;26:192–199. doi: 10.1016/j.clindermatol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Whittaker P, Kloner RA, Boughner DR, et al. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- 16.Rich L, Whittaker P. Collagen and picrosirius red staining: a polarized light assessment of fibrillar hue and spatial distribution. Braz J Morphol Set. 2005;22:97–104. [Google Scholar]
- 17.El-Domyati M, El-Ammawi TS, Medhat W, et al. Electro-optical synergy technique: a new and effective nonablative approach to skin aging. J Clin Aesthet Dermatol. 2010;3:22–30. [PMC free article] [PubMed] [Google Scholar]
- 18.El-Domyati M, El-Ammawi TS, Medhat W, et al. Effects of the Nd:YAG 1320-nm laser on skin rejuvenation: clinical and histological correlations. J Gosmet Laser Ther. 2011;13:98–106. doi: 10.3109/14764172.2011.586423. [DOI] [PubMed] [Google Scholar]
- 19.El-Domyati M, El-Ammawi TS, Medhat W, et al. Radiofrequency facial rejuvenation: evidence-based effect. J Am Acad Dermatol. 2011;64:524–535. doi: 10.1016/j.jaad.2010.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung HJ, Uitto J. Type VII collagen: the anchoring fibril protein at fault in dystrophic epidermolysis bullosa. Dermatol Clin. 2010;28:93–105. doi: 10.1016/j.det.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahoney MG, Brennan D, Starcher B, et al. Extracellular matrix in cutaneous ageing: the effects of 0.1% copper-zinc malonate-containing cream on elastin biosynthesis. Exp Dermatol. 2009;18:205–211. doi: 10.1111/j.1600-0625.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 22.El-Domyati M, El-Ammawi TS, Medhat W, et al. Multiple minimally invasive Erbium:YAG laser mini-peels for skin rejuvenation: an objective assessment. J Gosmet Dermatol. 2012;11:122–130. doi: 10.1111/j.1473-2165.2012.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournier N, Dahan S, Barneon G, et al. Nonablative remodeling: clinical, histologic, ultrasound imaging, and profilometric evaluation of a 1540nm Englass laser. Dermatol Surg. 2001;27:799–806. doi: 10.1046/j.1524-4725.2001.00355.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg DJ, Rogachefsky AS, Silapunt S. Non-ablative laser treatment of facial rhytides. A comparison of 1450nm diode laser treatment with dynamic cooling device as opposed to treatment with dynamic cooling alone. Lasers Surg Med. 2002;30:79–81. doi: 10.1002/lsm.10011. [DOI] [PubMed] [Google Scholar]
- 25.Grema H, Greve B, Raulin C. Facial rhytides—subsurfacing or resurfacing? A review. Lasers Surg Med. 2003;32:405–412. doi: 10.1002/lsm.10172. [DOI] [PubMed] [Google Scholar]
- 26.Doddaballapur S. Microneedling with dermaroller. J Clin Aesthet Dermatol. 2009;2:110–111. doi: 10.4103/0974-2077.58529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leheta T, El Tawdy A, Abdel Hay R, et al. Percutaneous collagen induction versus full-concentration trichloroacetic acid in the treatment of atrophic acne scars. Dermatol Surg. 2011;37:207–216. doi: 10.1111/j.1524-4725.2010.01854.x. [DOI] [PubMed] [Google Scholar]
- 28.Helfrich YR, Sachs DL, Voorhees JJ. Overview of skin aging and photoaging. Dermatol Nurs. 2008;20:177–183. [PubMed] [Google Scholar]
- 29.Majid I. Microneedling therapy in atrophic facial scars: an objective assessment. J Gutan Aesthet Surg. 2009;2:26–30. doi: 10.4103/0974-2077.53096. [DOI] [PMC free article] [PubMed] [Google Scholar]