Abstract
Background
Resting-state functional connectivity is a noninvasive, neuroimaging method for assessing neural network function. Altered functional connectivity among regions of the default-mode network have been associated with both nicotine and cannabis use; however, less is known about co-occurring cannabis and tobacco use.
Methods
We used posterior cingulate cortex (PCC) seed-based resting-state functional connectivity analyses to examine default mode network (DMN) connectivity strength differences between four groups: 1) individuals diagnosed with cannabis dependence who do not smoke tobacco (n=19; ages 20–50), 2) cannabis-dependent individuals who smoke tobacco (n=23, ages 21–52), 3) cannabis-naïve, nicotine-dependent individuals who smoke tobacco (n=24, ages 21–57), and 4) cannabis- and tobacco-naïve healthy controls (n=21, ages 21–50), controlling for age, sex, and alcohol use. We also explored associations between connectivity strength and measures of cannabis and tobacco use.
Results
PCC seed-based analyses identified the core nodes of the DMN (i.e., PCC, medial prefrontal cortex, inferior parietal cortex, and temporal cortex). In general, the cannabis-dependent, nicotine-dependent, and co-occurring use groups showed lower DMN connectivity strengths than controls, with unique group differences in connectivity strength between the PCC and the cerebellum, medial prefrontal cortex, parahippocampus, and anterior insula. In cannabis-dependent individuals, PCC-right anterior insula connectivity strength correlated with duration of cannabis use.
Conclusions
This study extends previous research that independently examined the differences in resting-state functional connectivity among individuals who smoke cannabis and tobacco by including an examination of co-occurring cannabis and tobacco use and provides further evidence that cannabis and tobacco exposure is associated with alterations in DMN connectivity.
Keywords: Cannabis, Dependence, Resting State Functional Connectivity, Nicotine
1. INTRODUCTION
Despite the acute and long-term negative effects of cannabis and tobacco use on health, cognition, and overall functioning, cannabis continues to be the most commonly used illicit drug (National Institute on Drug Abuse, 2014), and tobacco use continues to be the leading cause of preventable illness and death in the United States (Centers for Disease Control and Prevention, 2014). Cannabis and tobacco use disorders are chronic, relapsing disorders marked by compulsive drug-taking despite a wide range of negative consequences. Given that cannabis and tobacco use share several similarities, including their most common route of administration and associated cues, it is not surprising that cannabis and tobacco use commonly co-occur. According to recent survey data, approximately 36% of current adult cigarette smokers report cannabis use during the past 30 days, and 64% of current adult cannabis users report cigarette use during the past 30 days (United States Department of Health and Human Services, 2011). Co-occurring use of cannabis and tobacco is concerning, as individuals who smoke both cannabis and tobacco have a marked elevated risk of respiratory distress and reduced lung functioning compared to those who smoke cannabis or tobacco alone (Taylor et al., 2002). Further, cannabis users who also smoke tobacco have greater cannabis dependence, more psychosocial problems, and poorer cessation outcomes than those who use cannabis alone (Peters et al., 2012).
Although cannabis, tobacco, and their co-occurring use are prevalent, only one neuroimaging study has examined the similarities and differences in neural structure and functioning across individuals with cannabis use disorder (CUD), tobacco use disorder (TUD), and those who smoke cannabis and cigarettes concurrently (Wetherill et al., in press). Using voxel-based morphometry, Wetherill and colleagues (in press) compared gray matter volume across individuals with CUD, TUD, co-occurring use, and non-using, demographically-matched controls and found similarities and differences in gray matter volume within brain regions associated with reward and motivation. Specifically, individuals with CUD, TUD, and those with co-occurring use showed greater gray matter volume in the reward-related putamen; whereas, individuals with CUD and co-occurring use exhibited smaller thalamic gray matter volume compared to controls. Further, those with TUD and co-occurring use showed smaller cerebellar gray matter volume compared to controls. Taken together, these findings suggest significant similarities and differences in neural structure across individuals with CUD, TUD, and co-occurring use; however, additional research is needed to fully understand the unique and combined effects of these behaviors on brain structure and function.
Resting-state functional connectivity (rsFC) is a noninvasive, neuroimaging method that assesses intrinsic, dynamic interactions between groups of brain regions (e.g., neural networks) by identifying low-frequency, spontaneous fluctuations in the blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) signal (Biswal et al., 1995; Fox et al., 2005) between brain regions in the absence of explicit task demands, or “at rest”. Resting-state functional connectivity approaches have identified specific brain networks that correspond to those engaged during cognitive tasks (Smith et al., 2009) and those that predict behavioral performance (Kelly et al., 2008). As such, rsFC has become a popular tool that may provide insight into the dysfunctional neurocircuitry underlying addictive behaviors. Indeed, Sutherland and colleagues (2012) reviewed the existing rsFC literature and provided a potential network model of nicotine addiction, which may apply to other addictions, as well. The proposed model involved three distinct neural networks: 1) the default-mode network (DMN; Raichle et al., 2001), 2) the executive control network (ECN) (Seeley et al., 2007), and the salience network (SN; Seeley et al., 2007). The DMN is a prominent resting state network and is comprised of the posterior cingulate cortex (PCC), medial prefrontal cortex (mPFC), inferior parietal cortex, and temporal cortex (Greicius et al., 2003). This network is associated with self-referential processes, including memory, attention, and decision-making (Andrews-Hanna et al., 2010; Small et al., 2003). The ECN is comprised of lateral prefrontal and parietal regions and is involved in attention and decision-making processes. The SN includes the anterior cingulate cortex (ACC) and anterior insula and is thought to be involved in information processing by identifying the most salient information both internally and externally, and “toggling” between the DMN and ECN (Uddin et al., 2011).
Although research has explored the acute effects of Δ9-tetrahydrocannabinol (THC), the psychoactive component of cannabis (Gaoni and Mechoulam, 1971), and nicotine, the primary component of tobacco cigarettes, on rsFC (Hong et al., 2009; Klumpers et al., 2012; Lerman et al., 2014; Sutherland et al., 2013; Tanabe et al., 2011; van Hell et al., 2011), research examining rsFC of the DMN and its connections among individuals with CUD or TUD is sparse. One resting state fMRI study compared the DMN and other neural networks associated with self-referential processes (i.e., Insula network) in heavy cannabis users compared to healthy controls and found that cannabis users showed increased functional connectivity in the core nodes of the DMN and Insula networks and reduced functional connectivity in areas overlapping with other brain networks (Pujol et al., 2014). In a resting-state functional connectivity study among cigarette smokers, Weiland and colleagues (2014) compared DMN and ECN connectivity of smokers and non-smokers and found reduced connectivity strength within both the DMN and ECN of smokers relative to non-smokers (Weiland et al., 2014). Together, these findings indicate that cannabis and tobacco use have differential effects on the DMN. It is important to note, however, that Pujol and colleagues (2014) did not report or control for cigarette use among their heavy cannabis users, and Weiland and colleagues (2014) did not report or control for cannabis use among their smoking sample. Consequently, findings from these studies may be confounded by co-occurring use.
To date, there are no rsFC studies examining connectivity within and between the DMN among individuals who smoke cannabis and cigarettes concurrently, nor are there studies comparing connectivity between individuals with CUD, TUD, those who smoke cannabis and tobacco concurrently, and healthy controls. Given the high rates of co-occurring cannabis and cigarette use and paucity of research on rsFC among these groups, we aimed to (1) identify the differences in DMN connectivity among individuals who smoke tobacco cigarettes from those who smoke cannabis, (2) determine whether individuals with co-occurring cannabis and cigarette use show alterations in DMN connectivity, (3) explore whether DMN connectivity in cannabis users is associated with cannabis use (e.g., years of cannabis use), and (4) determine whether DMN connectivity in cigarette smokers is associated with tobacco use (i.e., pack years). Unlike previous studies, the current study compared DMN connectivity strength between cannabis-dependent individuals who do NOT smoke tobacco (Cs), cannabis-dependent individuals who smoke tobacco/cigarettes (CTs), nicotine-dependent, cannabis-naive individuals (Ts), and healthy, non-using controls (HCs). Given that the DMN is associated with memory and decision making (Andrews-Hanna et al., 2010; Small et al., 2003) and that cannabis and tobacco use, and withdrawal from these substances, alters these cognitive processes (Ashare et al., 2014; Solowij and Battisti, 2008), we hypothesized that Cs, CTs, and Ts would exhibit lower DMN connectivity strength compared to HCs. Further, we hypothesized lower DMN connectivity strength would correlate with cannabis use among Cs and CTs and nicotine exposure in CTs and Ts. We focused on resting brain connectivity between the PCC, a major connectivity hub in the brain whose connections define the DMN (Buckner et al., 2008; Fox and Raichle, 2007; Greicius et al., 2003; Zhu et al., 2013), and other brain regions throughout the brain.
2. MATERIAL AND METHODS
2.1. Participants and recruitment
All study procedures adhered to the Declaration of Helsinki and were approved by the University of Pennsylvania Institutional Review Board. Physically healthy individuals who are: 1) cannabis-dependent who do NOT smoke tobacco/cigarettes (C), 2) cannabis-dependent and smoke tobacco (CT), 3) cannabis-naïve, nicotine-dependent and smoke tobacco (T), and 4) non-using, cannabis- and tobacco-naive healthy controls (HC), were recruited via media advertisements and referrals. After completing an initial telephone screen, individuals received a description of their respective study, provided written informed consent, and completed a screening visit (i.e., physical examination and psychological assessment) to ensure that they fulfilled all study criteria. Exclusion criteria included current DSM-IV Axis I diagnoses (other than cannabis or nicotine dependence), lifetime history of head injury with loss of consciousness for more than 3 min, contraindications for magnetic resonance imaging, current treatment for cannabis dependence, clinically significant medical conditions, lifetime history of illicit drug use other than cannabis, and use of medication interacting with the central nervous system. Further details regarding the inclusion procedure are described in previous studies (Franklin et al., 2014; Wetherill et al., 2014). Approximately 45 minutes prior to scan acquisition, CTs and Ts were provided the opportunity to smoke a cigarette to ensure that they were not experiencing nicotine withdrawal symptoms during data acquisition. Self-report of last cannabis use in Cs and CTs was obtained (mean time since last use = 0.69 days, SD = 0.51). The final population meeting criteria for this study consists of 19 Cs (mean age = 28.0 years; SD 6.9), 23 CTs (mean age = 30.1 years; SD 8.9), 24 Ts (mean age = 36.0 years; SD 11.7), and 21 HCs (mean age = 30.6 years; SD 8.6). Demographic characteristics are shown in Table 1.
Table 1.
Demographic characteristics of cannabis-dependent individuals who do not smoke tobacco (C), cannabis-dependent individuals who smoke tobacco (CT), cannabis-naïve, nicotine-dependent individuals who smoke tobacco (T), and healthy controls (HC).
| C (n=19) | CT (n=23) | T (n=24) | HC (n=21) | p-values | |
|---|---|---|---|---|---|
| Sex, n(%), male | 10 (53) | 18 (78) | 14 (58) | 14 (67) | 0.32 |
| Age | 28 (7) | 30 (9) | 36 (12) | 31 (9) | 0.04a |
| Nicotine dependence (FTND) | - | 4.6 (1.2) | 4.3 (1.6) | - | 0.39b |
| Cigarettes per day | - | 7.2 (6.5) | 14.2 (5.3) | - | 0.001b |
| Pack years | - | 4.3 (4.8) | 11.6 (9.4) | - | 0.002b |
| Age of onset of cannabis use | 19 (5) | 17 (4) | - | - | 0.23c |
| Cannabis use, years | 9 (5) | 13 (8) | - | - | 0.08c |
| Cannabis use, past 30 days | 27 (4) | 25 (6) | - | - | 0.13c |
| Cannabis use, grams/week | 14 (11) | 20 (10) | - | - | 0.07c |
| Alcohol use, past 30 days | 2 (3) | 3 (4) | 2 (4) | - | 0.52d |
p-values for comparison between C and T groups
p-values for comparison between CT and T groups
p-values for comparison between C and CT groups
p-values for comparison between C, CT, and T groups
Notes: Means (SD); FTND, Fagerstrom Test of Nicotine Dependence
For all participants, urine drug screens verified the absence of illicit drugs (e.g., cocaine, opiates, amphetamines) and the absence of nicotine and its major metabolite, cotinine, in C and HC groups. The cannabis groups completed urine and saliva tests during the screening process to confirm the regular use of cannabis consumption. The Timeline Follow-Back (Sobell and Sobell, 1992) quantified substance use during the past 30 days, and the Addiction Severity Index (McLellan et al., 1992) assessed lifetime substance use. The Fagerstrom Test for Nicotine Dependence (FTND) (Fagerstrom and Schneider, 1989) assessed severity of nicotine dependence among CTs and Ts.
2.2. MR acquisition
Imaging data were acquired on a Siemens 3 Tesla Trio whole-body scanner (Erlangen, Germany) at the Hospital of the University of Pennsylvania using a product 8-channel head coil. A gradient-echo EPI sequence was used for resting-state BOLD fMRI data (repetition time (TR) = 2s, echo time (TE) = 24ms, field of view (FOV) = 220×220 mm, matrix 64×64×64, slice thickness = 4 mm, no inter-slice gap. Participants were instructed to lie still in the scanner and keep their eyes open. An eye-tracker outside of the scanner was used to monitor participants’ eyes and ensure that they remained awake. After the functional scans, high-resolution (1×1×1 mm3) T1-weighted anatomic images were obtained using a standard 3D MPRAGE sequence.
2.3. Data processing
Data were preprocessed and analyzed using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK) VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) and the REST 2.0 toolbox (http://resting-fmri.sourceforge.net/) implemented in MatlabR2013 (MathWorksInc., Natick, MA, USA). Images were corrected for timing differences between each slice and motion effects (six-parameter rigid body). Participants with a head motion greater than 2.0 mm maximum displacement in any direction or 2.0° of angular motion during the scan were not included in the current analysis. The remaining functional images were co-registered and smoothed using an isotropic Gaussian Kernel with a full-width at half-maximum (FWHM) of 4 mm, and then normalized to the standard Montreal Neurological Institute (MNI) space. Linear trends were removed. All functional volumes were finally band pass filtered at 0.01–0.08 Hz to reduce the low-frequency drift and physiological high-frequency respiratory and cardiac noise. Nuisance covariates including six head motion parameters, global mean signal, white matter signal, and cerebrospinal fluid (CSF) signal were regressed out before the seed-based functional connectivity (FC) analysis (Fair et al., 2008).
2.4. Data analysis
To assess DMN rsFC, a PCC seed was defined as a sphere with a radius of 10mm located in the MNI coordinate (0, −50, 31) (Zhu et al., 2013). For each individual subject, the mean BOLD fMRI signal time series was extracted and used as a regressor in the FC analysis. To assess connectivity strength, the correlation coefficients between the time series of the seed region and other brain areas were grouped into an individual FC map and transformed into z-scores through a Fisher’s r-to-z transformation to improve the normality of the correlation coefficients. Group-level ANCOVA analysis (F test) was performed on these z-transformed individual FC maps to investigate the main effect of group with age, sex, and alcohol use included as covariates of no-interest. Subsequent post hoc pairwise comparisons were conducted (e.g., Cs vs. CTs; Cs vs. Ts; Cs vs. HCs, etc.) to determine whether group differences were significant. Threshold was defined as whole brain p < 0.001, cluster-corrected at family-wise error (FWE) of p < 0.05 and k > 40 voxels. Values of the PCC BOLD time series correlation coefficients were also extracted for region of interest analyses, which were correlated with duration of cannabis use among Cs and CTs and nicotine exposure (i.e., pack years) among Ts and CTs using the IBM SPSS 19 software package (Arnouk, NY).
3. RESULTS
3.1. Demographic characteristics
As shown in Table 1, groups did not differ in sex. Groups did not differ in age, with the exception of Cs being significantly younger than Ts. Comparisons between CT and T groups revealed that T adults smoked more cigarettes per day and had greater pack years than CT adults, but did not differ in nicotine dependence (FTND). Cannabis-dependent adults (C vs CT groups) did not differ in age of cannabis use onset, cannabis use days (past 30 days), years of cannabis use, or amount of cannabis use (grams/week). C, CT, and T groups did not differ in alcohol use days (past 30 days).
3.2. Functional connectivity analysis
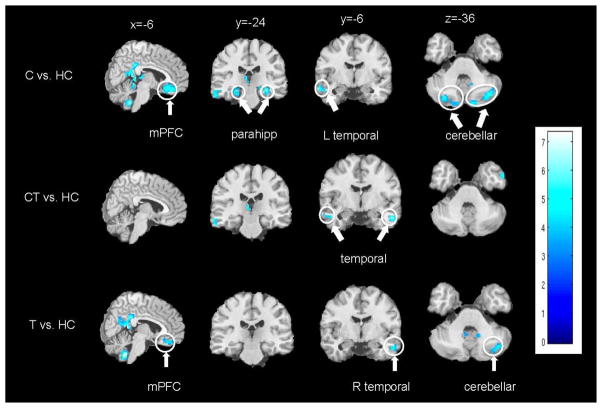
Functional connectivity analyses on the neuroimaging data using a PCC seed detected the core nodes of the DMN for all groups, including the PCC/retrosplenial cortex, inferior parietal cortex, mPFC, and temporal cortex. Significant group differences emerged between the PCC and regions of the temporal cortex, cerebellum, parahippocampus, and mPFC (Figures 1–2), with Cs, CTs, and Ts showing lower PCC-temporal cortex connectivity strength; Cs and Ts showing lower PCC-mPFC connectivity (i.e., ventral ACC/medial orbitofrontal cortex (mOFC)) and lower PCC-cerebellar connectivity (i.e., crus I/II); and Cs showing lower PCC-parahippocampal connectivity compared to HCs. Cs exhibited enhanced PCC-right anterior insula connectivity strength; whereas, Ts exhibited enhanced PCC-cerebellar connectivity (i.e., bilateral lobule VIIIB) and PCC-mPFC connectivity (i.e., bilateral frontal poles) compared to HCs. There were no significant differences between CTs compared Cs, CTs compared to Ts, or between Cs and Ts.
Figure 1.
Brain regions showing lower functional connectivity strength with the posterior cingulate cortex (PCC) compared to healthy controls (HC). Top row) Cannabis-dependent individuals who do not smoke tobacco (Cs) less than HCs. Middle row) Cannabis-dependent individuals who smoke tobacco (CTs) less than HCs. Bottom row) Nicotine-dependent, cannabis-naive individuals (Ts) less than HCs. Clusters of significant differences (p < 0.001, cluster-corrected at family-wise error (FWE) of p < 0.05 and k > 40 voxels) are displayed on representative sagittal, coronal, and axial slices overlain on the standard MNI brain. Right side of the brain is depicted on the right side. L = left; R = right; mPFC = medial prefrontal cortex; Parahipp = parahippocampus.
Figure 2.
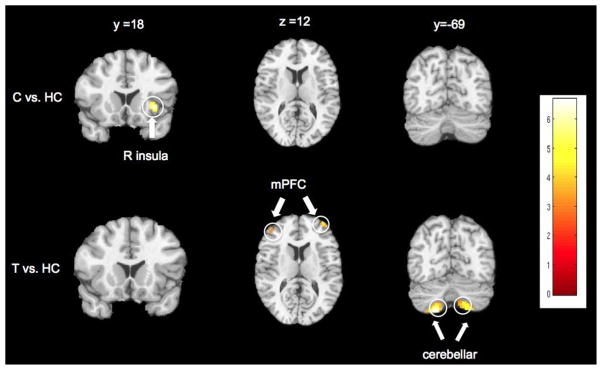
Brain regions showing enhanced functional connectivity strength with the posterior cingulate cortex (PCC) compared to healthy controls (HC). Clusters of significant differences (p < 0.001, cluster-corrected at family-wise error (FWE) of p < 0.05 and k > 40 voxels) are displayed on representative sagittal, coronal, and axial slices overlain on the standard MNI brain. Right side of the brain is depicted on the right side. C = cannabis-dependent individuals who do not smoke tobacco; T = nicotine-dependent, cannabis-naive individuals; R = right; mPFC = medial prefrontal cortex.
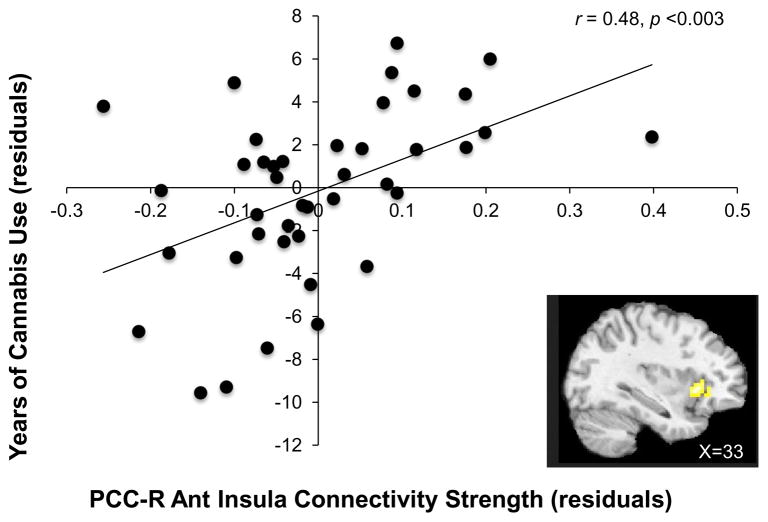
Among those who use cannabis (i.e., Cs and CTs), partial correlations examined potential associations between PCC connectivity and duration of cannabis use with age, sex, and tobacco use (i.e., pack years, a measure of lifetime cigarette consumption) as covariates. A significant correlation was found between PCC-right anterior insula connectivity and years of cannabis use (r = 0.48, p = 0.003, Figure 3) indicating that the longer a person has been smoking cannabis, the stronger the connectivity between the PCC and right anterior insula. Similar partial correlation analyses were conducted for those who smoke tobacco (i.e., Ts and CTs) by exploring associations between PCC connectivity and pack years with age, sex, and cannabis use (i.e., grams per week) as covariates; however, no significant correlations were found between PCC connectivity and pack years.
Figure 3.
Correlation between PCC-right anterior insula connectivity strength and years of cannabis use controlling for age, sex, and pack years (i.e., a measure of nicotine exposure).
4. DISCUSSION
The current study provides evidence that Cs, CTs, and Ts show differing patterns of DMN connectivity compared to HCs. To explore DMN resting-state functional connectivity, we used PCC seed-based rsFC analyses and found the core nodes of the DMN for all groups, including the PCC/retrosplenial cortex, inferior parietal cortex, mPFC, and temporal cortex, yet significant group differences emerged. Compared to controls, Cs, CTs, and Ts exhibited reduced DMN connectivity strength, with similar reductions in connectivity strength between the PCC and the temporal cortex and unique differences in connectivity strength between the PCC and other brain regions among Cs and Ts; however, CTs did not. Cs showed lower connectivity strength between the PCC and the mPFC, cerebellar regions, and parahippocampus, yet greater connectivity between the PCC and right anterior insula. Ts exhibited lower connectivity strength between the PCC and the cerebellar regions, yet enhanced connectivity strength between the PCC and regions of the cerebellum and mPFC compared to HCs. In Cs and CTs, PCC resting-state connectivity with the right anterior insula correlated with duration of cannabis use. Among CTs and Ts, there were no significant correlations between nicotine exposure and PCC connectivity.
As hypothesized, compared to HCs, Cs, CTs, and Ts showed alterations in DMN connectivity; however, direct comparisons between Cs, CTs, and Ts revealed no differences. These finding are consistent with previous studies among other drug using populations, including heroin (Ma et al., 2011), cocaine (Ding and Lee, 2013), and alcohol (Muller-Oehring et al., 2014), and provide additional support for a general addiction-related disruption of DMN connectivity. As previously mentioned, the DMN is involved in self-referential processes and how these internal processes relate to the external environment (Sutherland et al., 2012); thus, abnormalities within the DMN and its interactions with other brain networks may underlie the cognitive and behavioral impairments observed among substance users. Given the consistencies between our study and research among other substance-using populations, these findings could reflect an “addiction vulnerable” brain state wherein addicted individuals possess neural vulnerabilities of the DMN and other neural networks that result in impairments in executive control, emotion regulation, and reward processing. It is important to note, however, that these neural vulnerabilities may exist prior to the onset of substance use.
Lower functional connectivity strength between the PCC and regions of the cerebellum were observed in Cs and Ts. Despite the fact that the cerebellum has been primarily associated with motor functions, research suggests that the cerebellum may also be involved in non-motor functions, such as executive control, salience detection, and memory/self-reflection (Habas et al., 2009; Stoodley and Schmahmann, 2009). Indeed, a recent rsFC study using independent component analysis found that specific regions within the cerebellum are involved in specific cognitive tasks and networks (Habas et al., 2009). In both C and T groups, lower connectivity was observed between the PCC and bilateral regions of crus I and crus II. Interestingly, recent functional connectivity analyses using the crus I as a seed region found significant correlated activity between the crus I and the mPFC, the inferior parietal cortex, and the PCC (Krienen and Buckner, 2009; Wang et al., 2014). Further, crus I and II abnormalities have been associated with deficits in integrating and regulating emotional and cognitive functions (Igloi et al., 2014; Riva et al., 2013). As such, our findings are consistent with previous studies showing crus involvement in the spontaneous brain activity of the DMN (Krienen and Buckner, 2009; Wang et al., 2014), and weaker connectivity strength between the PCC and Crus I/II could underlie the cognitive and emotional deficits associated with cannabis use disorder and tobacco use disorder. It is important to note that we did not observe differences in PCC-Crus connectivity strength among CTs relative to HCs; however, differences emerged when the statistical threshold was reduced (i.e., p < 0.005, uncorrected).
Another DMN region showing altered connectivity strength with the PCC is the mPFC. Among Cs, PCC-mPFC connectivity strength was lower compared to HCs; whereas, Ts showed enhanced PCC-mPFC connectivity strength in some mPFC regions and lower connectivity strength in other mPFC regions compared to HCs. Similar to the cerebellum, the mPFC is subdivided into different regions involved in different aspects of cognition and emotion. Cs and Ts showed lower connectivity strength between the PCC and the ventral ACC/mOFC. The ventral ACC/mOFC is a brain region with dense connections to emotional regions (e.g., amygdala, insula) involved in emotion regulation and affective processing (Margulies et al., 2007). Specifically, the ventral ACC/mOFC is believed to receive reinforcement expectancy information from these limbic structures (e.g., amygdala) involved in processing reinforcement (Blair, 2007), and as such, is engaged in identifying self-relevant information and assessing stimuli salience (Li et al., 2014). Therefore, lower connectivity between the PCC and the ventral ACC/mOFC suggests an effect of cannabis use and cigarette smoking on information processing and decision-making. Conversely, Ts showed enhanced connectivity strength between the PCC and other regions of the mPFC (i.e., bilateral frontopolar cortex). The frontopolar cortex, like other regions of the DMN, is believed to be involved in cognitive tasks that require processing of self-generated information (Christoff and Gabrieli, 2000). Given that the frontal pole is the largest architectonic area in the human frontal lobe, Moayedi and colleagues (2014) conducted a connectivity-based parcellation study of the frontal pole and identified two structural and functional subregions: the lateral and medial frontal poles. In this study, Ts showed connectivity strength differences in frontopolar regions consistent with the lateral frontal poles. The lateral frontal poles are connected to nodes of the ECN, associated with attention and working memory (Moayedi et al., 2014). As such, our finding of enhanced functional connectivity strength between the DMN and regions of the ECN seems inconsistent with findings indicating weaker DMN-ECN connectivity in cigarette smokers (Lerman et al., 2014); however, it is important to note that the study showed decreased coupling between the DMN and ECN during abstinence/withdrawal. In the current study, Ts smoked a cigarette approximately 45 minutes prior to the scanning session, and were therefore in a sated state. Thus, enhanced connectivity between the PCC and the bilateral frontopolar cortex among Ts compared to HCs could be due to nicotine’s direct effects, which have been shown to enhance attention and cognitive function (Heishman et al., 2010).
Consistent with studies demonstrating the negative effects of cannabis use and THC on the hippocampus/parahippocampus (Lawston et al., 2000; Scallet, 1991), Cs exhibited lower PCC-parahippocampal coupling compared to HCs. The inclusion of the hippocampus formation as part of the DMN has been inconsistent across studies (Greicius et al., 2003; Raichle and Snyder, 2007). This inconsistency may be related to the fact that hippocampal regions seem to lack a direct functional connection with the cortical DMN nodes, but are indirectly connected with the PCC via the parahippocampus (Ward et al., 2014). Given that the hippocampus and parahippocampus are involved in learning and memory, reduced connectivity strength between the PCC and parahippocampus of Cs may contribute to the memory deficits commonly associated with cannabis use. Unfortunately, we did not collect memory-related behavioral data to explore this potential association; thus, future studies are warranted.
Compared to HCs, Cs exhibited enhanced connectivity strength between the PCC and the right anterior insula. In addition, PCC-right anterior insula connectivity strength correlated with duration of cannabis use. This finding is consistent with the Pujol and colleagues study (2014) showing that average joints per year positively correlated with PCC-right anterior insula connectivity strength. The anterior insula is a key component of the SN involved in interoceptive and visceral awareness (Caseras et al., 2013; Critchley et al., 2004) and functions along with the ACC and amygdala in integrating external and internal stimuli (Sutherland et al., 2012). Given that the SN has been proposed to influence information processing by identifying the most relevant stimuli (Seeley et al., 2007) and cannabis cues are particularly important salient cues for cannabis users, these findings could suggest that that the enhanced connectivity between the PCC and anterior insula could underlie cannabis user’s heightened responsivity to cannabis cues, or attentional bias for cannabis cues.
Although Cs and Ts showed DMN connectivity strength differences compared to HCs, CTs did not exhibit unique differences in PCC connectivity strength. As such, it appears that co-occurring cannabis and tobacco use does not have an additive effect on DMN connectivity strength. Further, given that the Cs and Ts showed additional connectivity differences compared to HCs, it is possible that co-occurring cannabis and tobacco use could be neuroprotective. We acknowledge that this interpretation of our findings is speculative; however, research indicates that components of cannabis and tobacco have neuroprotective effects through endocannabinoid signaling (Ferrea and Winterer, 2009; Sarne and Mechoulam, 2005). Thus, additional research on co-occurring cannabis and tobacco use is needed and should explore both the neurotoxic and neuroprotective effects of co-occurring use.
4.1. Strengths and Limitations
This study has several important strengths and limitations. It is the first study to explore the differences in resting-state functional connectivity strength among adults who use cannabis, tobacco, and cannabis and tobacco concurrently. The groups were well-matched on demographic characteristics, and by including Cs, CTs, Ts, and HCs, we examined the differences in DMN connectivity strength between these groups. This cross-sectional study design prohibits our ability to dissociate causal effects of cannabis and tobacco smoking from predisposing biological factors. Similarly, behavioral measures, such as impulsivity or sensation seeking, may differ between groups and contribute to these findings, and consequently, future studies are warranted. It is also important to note that analyses focused on a single seed in the DMN, and as such, findings are limited to PCC functional connectivity. To address this limitation, future research should explore additional within and between network analyses. Finally, our sample size also precludes us from examining how other factors, such as sex and genetic vulnerabilities, may influence these findings.
4.2. Conclusion
This study provides new information on the potential effects of cannabis, cigarettes, and co-occurring cannabis and cigarette smoking on resting-state functional connectivity. In general, Cs, CTs, and Ts have reduced connectivity strength in the DMN compared to HCs; however, unique differences in reductions and enhancements across groups emerged. In addition, PCC-anterior insula correlation strength correlated with duration of cannabis use suggesting that the longer an individual has smoked cannabis, the stronger their PCC-anterior insula connectivity. Although studies are needed, this study extends previous studies that independently examine the effects of cannabis or tobacco use on resting-state connectivity by including an examination of co-occurring cannabis and tobacco use, exclusive use of one or the other, and potential correlations between connectivity strength and substance use.
Highlights.
We examine resting state functional connectivity of the default mode network.
We compare connectivity strength differences.
All of the smoking groups show lower connectivity strengths than controls.
Default mode network-insula connectivity strength correlates with cannabis use.
Acknowledgments
Role of funding source
This study was supported by grants received from the Pennsylvania Department of Health Commonwealth Universal Research Enhancement program awarded to ARC, the National Institutes of Health (R21 DA032022 and R01 HL102119 to HR; R01 DA029845 and R01 DA030394 to TRF), and a pilot grant from the Institute for Translational Medicine and Therapeutics of the University of Pennsylvania awarded to HR. These funding sources had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the manuscript for publication.
Footnotes
Contributors
Reagan R. Wetherill was primarily responsible for the design of the study, assisting in the data collection analysis, and writing the first draft of the manuscript. Zhuo Fang and Kanchana Jagannathan assisted with data analysis. Hengyi Rao was responsible for programming of tasks and assisted with data analysis. Anna Rose Childress and Teresa R. Franklin provided assistance in study design, interpretation of findings and feedback on drafts of the manuscript. All authors have read and approve the final version of the manuscript.
Conflict of interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, Lerman C. Cognitive function during nicotine withdrawal: Implications for nicotine dependence treatment. Neuropharmacology. 2014;76(Pt B):581–591. doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Blair RJ. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Caseras X, Murphy K, Mataix-Cols D, Lopez-Sola M, Soriano-Mas C, Ortriz H, Pujol J, Torrubia R. Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Hum Brain Mapp. 2013;34:1220–1229. doi: 10.1002/hbm.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2005–2012. MMWR. 2014;63:29–34. [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrieli JD. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Ding X, Lee SW. Cocaine addiction related reproducible brain regions of abnormal default-mode network functional connectivity: a group ICA study with different model orders. Neurosci Lett. 2013;548:110–114. doi: 10.1016/j.neulet.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci USA. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrea S, Winterer G. Neuroprotective and neurotoxic effects of nicotine. Pharmacopsychiatry. 2009;42:255–265. doi: 10.1055/s-0029-1224138. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, Rao H, Childress AR. The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One. 2014;9:e104102. doi: 10.1371/journal.pone.0104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish. J Am Chem Soc. 1971;93:217–224. doi: 10.1021/ja00730a036. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210:453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi K, Doeller CF, Paradis AL, Benchenane K, Berthoz A, Burgess N, Rondi-Reig L. Interaction between hippocampus and cerebellum crus I in sequence-based but not place-based navigation. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Klumpers LE, Cole DM, Khalili-Mahani N, Soeter RP, Te Beek ET, Rombouts SA, van Gerven JM. Manipulating brain connectivity with delta(9)-tetrahydrocannabinol: a pharmacological resting state FMRI study. NeuroImage. 2012;63:1701–1711. doi: 10.1016/j.neuroimage.2012.07.051. [DOI] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawston J, Borella A, Robinson JK, Whitaker-Azmitia PM. Changes in hippocampal morphology following chronic treatment with the synthetic cannabinoid WIN 55,212–2. Brain Res. 2000;877:407–410. doi: 10.1016/s0006-8993(00)02739-6. [DOI] [PubMed] [Google Scholar]
- Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71:523–530. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mai X, Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front Hum Neurosci. 2014;8:74. doi: 10.3389/fnhum.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu XM, Li N, Wang CX, Zhang H, Qian RB, Xu HS, Hu X, Zhang DR. Abnormal brain default-mode network functional connectivity in drug addicts. PloS one. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Moayedi M, Salomons TV, Dunlop KA, Downar J, Davis KD. Connectivity-based parcellation of the human frontal polar cortex. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, Schulte T. The resting brain of alcoholics. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse. Marijuana. 2014 Retrieved from http://www.drugabuse.gov/publications/drugfacts/marijuana on December 30, 2014.
- Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addiction. 2012;107:1404–1417. doi: 10.1111/j.1360-0443.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C, Crippa JA, Fagundo AB, Deus J, de la Torre R, Nogue S, Farre M, Torrens M, Martin-Santos R. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 2014;51:68–78. doi: 10.1016/j.jpsychires.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- Riva D, Annunziata S, Contarino V, Erbetta A, Aquino D, Bulgheroni S. Gray matter reduction in the vermis and CRUS-II is associated with social and interaction deficits in low-functioning children with autistic spectrum disorders: a VBM-DARTEL Study. Cerebellum. 2013;12:676–685. doi: 10.1007/s12311-013-0469-8. [DOI] [PubMed] [Google Scholar]
- Sarne Y, Mechoulam R. Cannabinoids: between neuroprotection and neurotoxicity. Curr Drug Targets CNS Neurol Disord. 2005;4:677–684. doi: 10.2174/156800705774933005. [DOI] [PubMed] [Google Scholar]
- Scallet AC. Neurotoxicology of cannabis and THC: a review of chronic exposure studies in animals. Pharmacol Biochem Behav. 1991;40:671–676. doi: 10.1016/0091-3057(91)90380-k. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. NeuroImage. 2003;18:633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, N.J: 1992. pp. 41–72. [Google Scholar]
- Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1:81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74:538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Nyberg E, Martin LF, Martin J, Cordes D, Kronberg E, Tregellas JR. Nicotine effects on default mode network during resting state. Psychopharmacology. 2011;216:287–295. doi: 10.1007/s00213-011-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DR, Fergusson DM, Milne BJ, Horwood LJ, Moffitt TE, Sears MR, Poulton R. A longitudinal study of the effects of tobacco and cannabis exposure on lung function in young adults. Addiction. 2002;97:1055–1061. doi: 10.1046/j.1360-0443.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar KS, Ryali S, Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J Neurosci. 2011;31:18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. National Survey on Drug Use and Health, 2012. Substance Abuse and Mental Health Services and Inter-university Consortium for Political and Social Research; Ann Arbor: 2012. ICPSR34933-v2. [Google Scholar]
- van Hell HH, Bossong MG, Jager G, Kristo G, van Osch MJ, Zelaya F, Kahn RS, Ramsey NF. Evidence for involvement of the insula in the psychotropic effects of THC in humans: a double-blind, randomized pharmacological MRI study. Int J Neuropsychopharmacol. 2011;14:1377–1388. doi: 10.1017/S1461145711000526. [DOI] [PubMed] [Google Scholar]
- Wang L, Zou F, Shao Y, Ye E, Jin X, Tan S, Hu D, Yang Z. Disruptive changes of cerebellar functional connectivity with the default mode network in schizophrenia. Schizophr Res. 2014;160:67–72. doi: 10.1016/j.schres.2014.09.034. [DOI] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, Van Dijk KR, Hedden T, Sperling RA. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp. 2014;35:1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, Welsh RC, Hutchison KE. Reduced executive and default network functional connectivity in cigarette smokers. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Childress AR, Jagannathan K, Bender J, Young KA, Suh JJ, O’Brien CP, Franklin TR. Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis-dependent individuals. Psychopharmacology. 2014;231:1397–1407. doi: 10.1007/s00213-013-3342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Hager N, Childress AR, Rao H, Franklin TR. Cannabis, cigarettes, and their co-occurring use: disentangling differences in gray matter volume. Int J Neuropsy. doi: 10.1093/ijnp/pyv061. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Fang Z, Hu S, Wang Z, Rao H. Resting state brain function analysis using concurrent BOLD in ASL perfusion fMRI. PloS One. 2013;8:e65884. doi: 10.1371/journal.pone.0065884. [DOI] [PMC free article] [PubMed] [Google Scholar]