Figure 1.
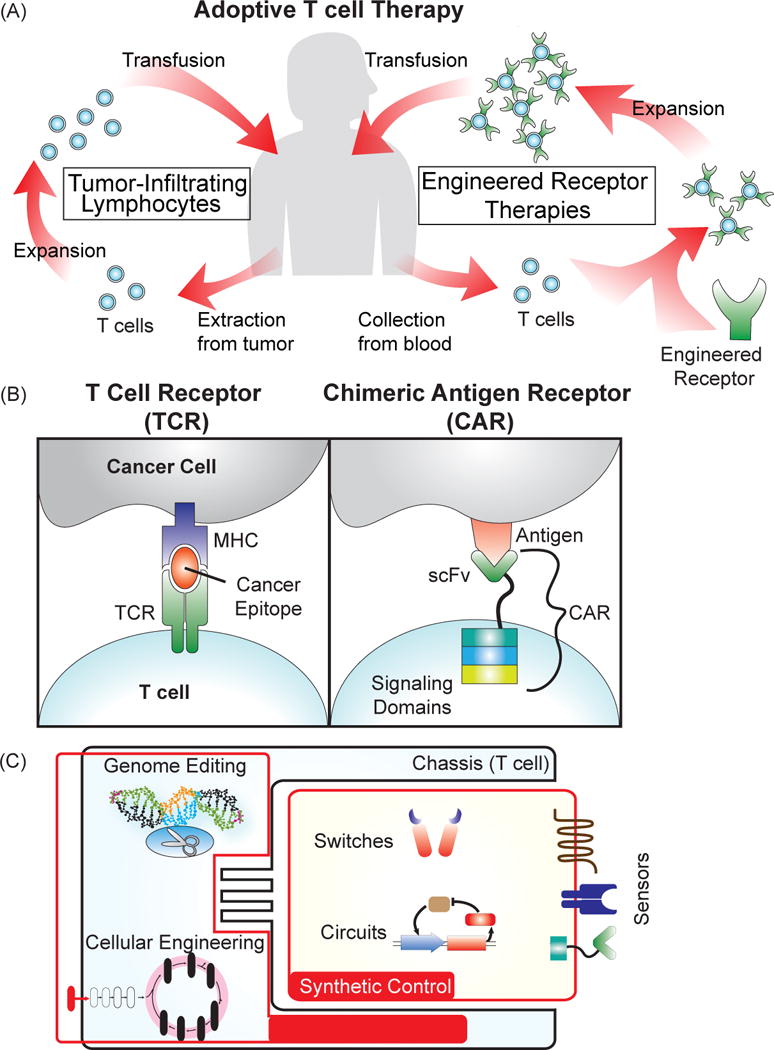
Adoptive T cell therapy for cancer treatment. (A) Several approaches for the adoptive transfer of a patient’s own T cells for cancer therapy. Tumor-infiltrating lymphocytes (TILs) involve extraction of T cells directly from the tumor, ex vivo expansion, and then transfusion back into the patient. For engineered receptor therapies, T cells are collected from the blood, genetically modified to express a cancer-targeting receptor, expanded, and then transfused back into the patient. (B) Receptors engineered to target cancer cells. T cell receptors (TCRs) naturally recognize protein epitopes presented by the major histocompatibility complex of a target cell. Engineering a TCR to detect cancer epitopes “teaches” the T cell to detect cancer cells. Chimeric antigen receptors (CARs) are composed of a single-chain variable fragment (scFv) from an antibody fused to intracellular T cell signaling domains that trigger activation and proliferation of the T cell. CARs recognize markers expressed at the surface of a cell, and by choosing a cancer-specific scFv, can be made to trigger killing of the cancer cell upon binding to the target antigen. (C) Engineering T cells for improvement of adoptive T cell therapy. Generating novel receptors and circuits can enable increased control over cell-based therapies, and techniques to engineer the chassis, such as genome editing and cellular engineering, can drive the development of more powerful treatments.
