Figure 2.
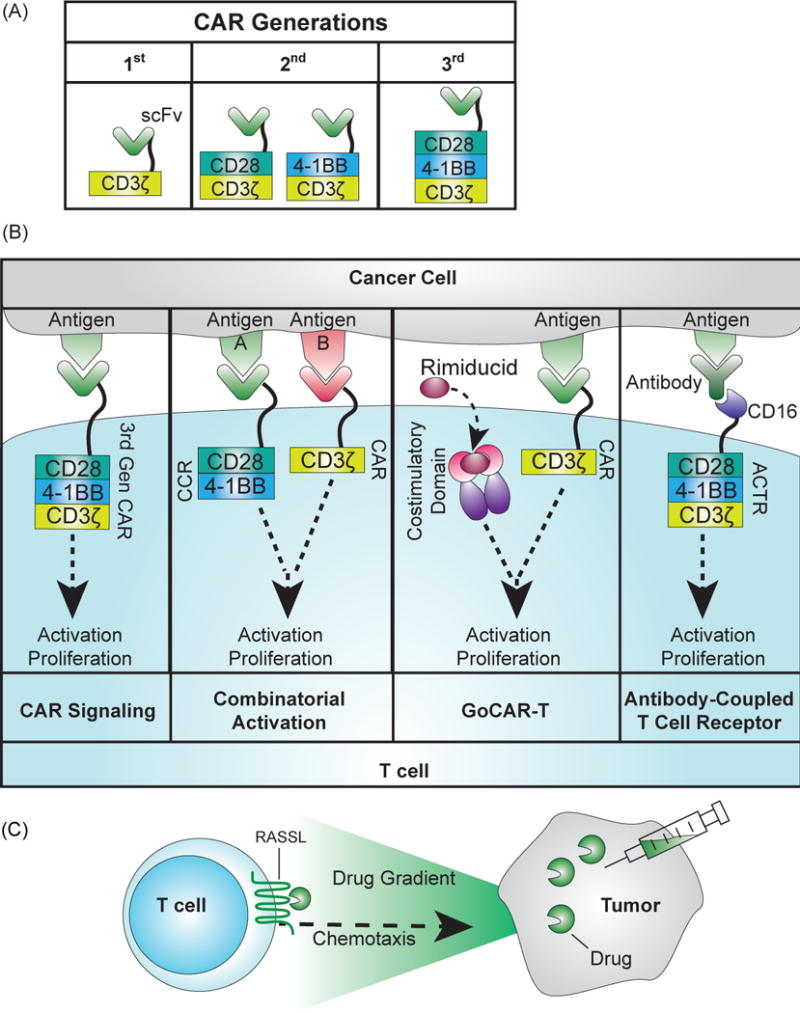
Receptor engineering for adoptive T cell therapy. (A) Development of CARs across three generations. The first generation CAR consisted of a scFv fused to the activating CD3ζ domain that drove activation upon antigen binding, but did not lead to sufficient persistence in clinical trials [116]. Second and third generation CARs have included the intracellular portions of proliferative signaling proteins, CD28 or 4-1BB. (B) Signaling of novel receptors for adoptive T cell therapy. First panel: In second and third generation CARs, binding of the antigen to the scFv triggers proliferation and activation of the T cell. Second panel: With combinatorial activation, binding to the CAR and chimeric costimulatory receptor (CCR) is required to drive both activation and proliferation of the T cell [48]. Third panel: The GoCAR-T system requires binding to the target antigen for activation, but it also requires the addition of the drug rimiducid to dimerize the co-stimulatory domain for activation and proliferation [50] (http://www.bellicum.com/technology/gocart/). Fourth panel: Antibody-coupled T cell receptors (ACTR) express a CD16 domain at the T cell surface instead of a scFv. CD16 binds to antibodies, and choosing antibodies that bind to the surface of cancer cells will drive T cell activity against the cancer cell [51]. (C) Engineered receptor for chemotaxis [59]. RASSLs, an engineered G protein-coupled receptor, are activated at the surface of the T cell by a drug and drive chemotaxis of the cell along the drug gradient. Adding the drug to a tumor can direct T cells towards the tumor site.
