Figure 4.
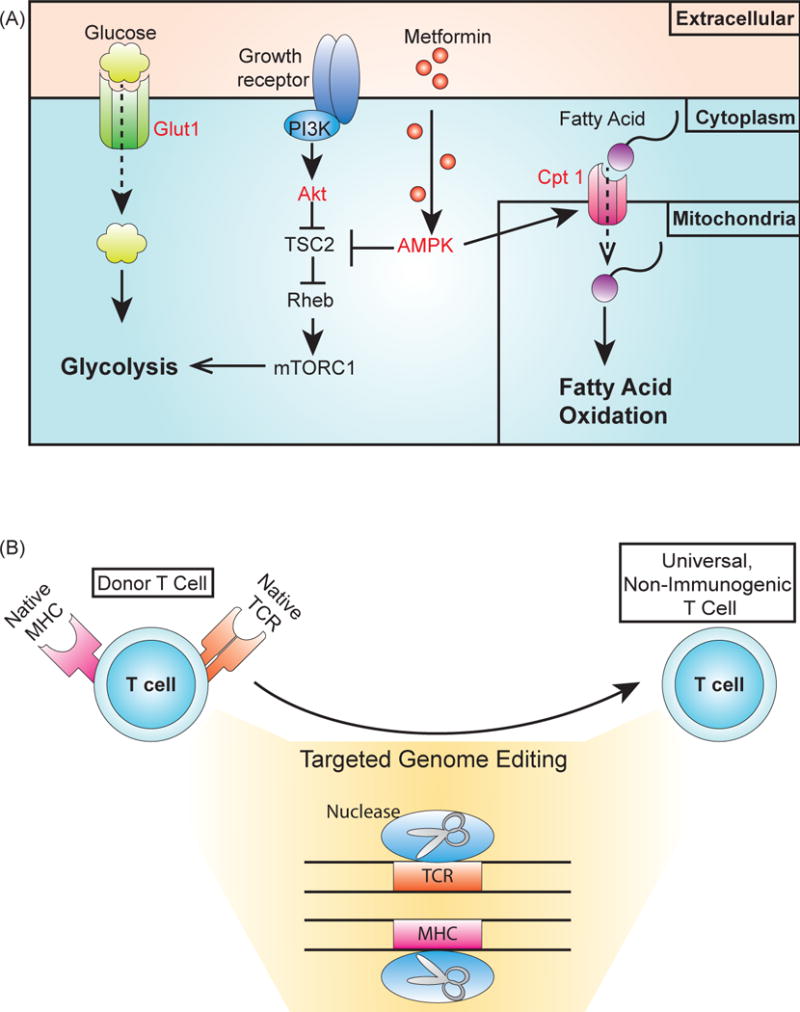
Cellular engineering and genome editing for chassis engineering. (A) Targets to bias T cells towards a naïve state or development of the memory T cell phenotype. Limiting glycolysis can prevent T cells from differentiating into effector cells promote memory T cell development [75, 79]. In addition, memory T cell development can be increased by promoting fatty acid oxidation. Different components of these metabolic pathways can be targeted for these aims (labeled in red). Reducing expression of Glut1, the glucose transporter limits the intake of glucose, while expressing an Akt inhibitor limits glycolysis due to Akt activation of mTOR through TSC2 and Rheb signaling [76, 77, 117]. Fatty acid oxidation can be promoted by overexpressing the fatty acid transporter Cpt-1 [79]. Activating AMPK with the drug metformin promotes fatty acid activation, both by repressing components involved in activating glycolysis and by indirectly overexpressing Cpt-1 [79]. (B) Genome editing for the production of an allogeneic, non-immunogenic T cell. Using targeted nucleases to disrupt expression of the T cell receptor (TCR) renders the T cell unable to detect targets until modification with an engineered receptor [87]. This process could be used to produce universal T cells from healthy donors that could be stored in a “T cell bank” for use in patients, as the lack of endogenous TCR expression would prevent graft-versus-host disease (GVHD). The expression of CARs and genetic circuits can involve the expression of components that elicit an immunogenic response. Disrupting major histocompatibility complex (MHC) expression prevents the T cell from presenting epitopes from these components, which would reduce the risk of an immunogenic response [88].
