Abstract
Background
Recent human studies confirm animal research showing that nicotine enhances reinforcement from rewards unrelated to nicotine. These effects of acute nicotine via tobacco smoking may also occur when consumed from non-tobacco products.
Methods
We assessed acute effects of nicotine via electronic cigarettes (“e-cigarettes”) on responding reinforced by music, video, or monetary rewards, or for no reward (control). In a fully within-subjects design, adult dependent smokers (N=28) participated in three similar experimental sessions, each following overnight abstinence (verified by CO≤10 ppm). Varying only in e-cigarette condition, sessions involved controlled exposure to a nicotine (labeled “36 mg/ml”) or placebo (“0”) e-cigarette, or no e-cigarette use. A fourth session involved smoking one’s own tobacco cigarette brand after no abstinence, specifically to compare responses under typical nicotine satiation with these acute e-cigarette conditions after abstinence.
Results
Reinforced responding for video reward, but not the other rewards, was greater due to use of the nicotine versus placebo e-cigarette (i.e., nicotine per se), while no differences were found between the placebo e-cigarette and no e-cigarette conditions (i.e., e-cigarette use per se). For nicotine via tobacco smoking, responding compared to the nicotine e-cigarette was similar for video but greater for music, while both video and music reward were enhanced relative to the non-nicotine conditions (placebo and no e-cigarette).
Conclusions
Acute nicotine from a non-tobacco product has some reinforcement enhancing effects in humans, in a manner partly consistent with nicotine via tobacco smoking and perhaps contributing to the rising popularity of nicotine e-cigarette use.
Keywords: Nicotine, E-cigarettes, Reinforcement enhancement, Sensory reward, CReSS, Smoking
1. INTRODUCTION
Recent human studies have confirmed animal models showing acute effects of nicotine in enhancing reinforcement from rewards made available independent of nicotine intake (Perkins and Karelitz, 2013a, 2013b, 2014). However, nicotine dosing in this human research was manipulated via controlled tobacco smoking, and we are not aware of any human research examining reinforcement enhancing effects of nicotine administered via non-tobacco means. Testing this effect with non-smoked nicotine would help isolate reinforcement enhancing effects due to nicotine per se and perhaps more closely match the manner of nicotine administration used in the corresponding animal studies (e.g., Caggiula et al., 2009).
A non-smoked nicotine product rapidly rising in prevalence, both among tobacco users and non-users, is electronic cigarettes (“e-cigarettes”; e.g., McMillen et al., 2015). Although non-nicotine e-cigarettes are marketed, e-cigarettes containing nicotine are far more common (Etter and Eissenberg, 2015). Actual nicotine absorption varies across brands and use patterns (Vansickel and Eissenberg, 2013; Goniewicz et al., 2013), but most appear to contain amounts comparable to the labeled contents (e.g., Davis et al., 2015; Etter et al., 2013) and can reach peak plasma nicotine levels within 5 mins of use (e.g., Vansickel and Eissenberg, 2013; Dawkins and Corcoran, 2014; Hajek et al., 2015). Some research shows rapid effects of nicotine from e-cigarettes on cognitive function, subjective effects, and other acute responses (Dawkins et al., 2013; Dawkins and Corcoran, 2014). Thus, nicotine from e-cigarettes may have reinforcement enhancing effects, as with tobacco smoking, which could help contribute to their popularity.
The current study assessed reinforcement enhancing effects of acute nicotine from the non-smoked product of e-cigarettes. Specifically, those containing nicotine were compared with non-nicotine (placebo) e-cigarettes. Consistent findings in our human studies of nicotine via tobacco use (e.g., Perkins and Karelitz, 2013a, 2014), and the auditory and visual (e.g., tones, lights) types of reinforcers enhanced by nicotine in animal research (see Donny et al., 2003; Caggiula et al., 2009; Raiff and Dallery, 2009), strongly suggest that nicotine only selectively enhances reinforcement from non-drug rewards. Thus, based on this research, we hypothesized nicotine from e-cigarettes would increase reinforced responding for qualitatively different types of rewards, rather than in non-specific fashion. Nicotine would enhance music or video rewards—those perhaps “sensory” in nature (see Fowler, 1971)—but not a “non-sensory” monetary reward or responding during a no reward control condition. A no e-cigarette session after overnight abstinence was included to confirm lack of effects on responding due to the behavior of using an e-cigarette, regardless of nicotine.
Separately, for comparison only, a fourth session involved intermittent smoking of one’s preferred tobacco brand (unblinded) after no overnight abstinence. This session was added to explore the extent to which modest acute nicotine e-cigarette use after abstinence would match reinforced responding under typical non-abstinent smoking conditions. We recently found no such effects from minimal nicotine via smoking (one-half cigarette), despite significant enhancement of reinforcement from moderate nicotine (a full cigarette; Perkins and Karelitz, 2013b). This observation raises the question of what amount, in addition to what manner, of acute nicotine intake is needed to enhance reinforcement.
2. METHODS
2.1 Participants
Participants (N=28; 12 men, 16 women) were adults who smoked ≥ 10 cigarettes per day for at least one year and met DSM-IV criteria for nicotine dependence (APA, 1994). Respective mean (± SD) smoking characteristics were 15.7±5.2 cigarettes/day, 4.5±2.0 on the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al., 1991), and nicotine yield of their preferred brand of 1.1±0.1 mg (non-menthol for 19, or 68%, and menthol for the other 9, or 32%). They were 26.5±6.6 years of age, and men and women did not differ on these smoking characteristics or age. They mostly self-identified as Caucasian (75.0%), with 21.4% African American, and 3.6% more than one ethnicity. Those currently taking medications to treat serious psychological problems (e.g., psychosis, major depression) were excluded.
2.2 Electronic Cigarettes
Aside from the non-abstinent tobacco smoking session, subjects abstained overnight prior to intermittent e-cigarette use during two sessions and remained abstinent from both products in a fourth session (as described below in Procedures). The e-cigarettes were obtained from PrimeVapor LLC (Pleasant Prairie WI), labeled as containing 36 mg or 0 mg nicotine content per ml of liquid in vegetable glycerin (www.primevapor.com), with pre-filled cartridges. The nicotine and placebo versions we used, “Rawhide Red (Tobacco)” for non-menthol and “Freeport (Menthol)” for menthol, were those expected to most closely match typical tobacco flavorings. They were provided with a KR808D-1 Type automatic E-cigarette battery. (See 2.4.2 Puff Topography Control, below.)
2.3 Reinforcement Task
Reinforced responding was assessed, as in our prior studies of acute nicotine effects on enhancing reinforcement (e.g., Perkins and Karelitz, 2013a), with a simple computer task (“Applepicker”; Norman and Jongerius, 1985). Briefly, subjects use the arrow keys to move a cursor around a “field” on a monitor and press a button (one “response”) when the cursor lands on one of the “trees” (circles) to look for “apples.” Number of responses required to find an “apple” (and earn one unit of the designated reinforcer; see below) constitutes the reinforcement schedule, which here was a progressive ratio incrementing by 50% after each completed ratio (i.e., PR50%), beginning at FR10.
Also, as in our prior studies, the four 15-min task trials per session differed in the single reward available each trial, with rewards presented over the session in counter-balanced order between subjects, and the same order used across sessions for the same subject. Rewards (and unit of each earned reinforcer) were: 1) music (playing 30 sec of preferred music through headphones), 2) video (playing 30 sec of preferred video, shown on monitor next to task “field”), 3) money (counter on monitor incrementing by $.10), and 4) no reward (control). Reward delivery was immediate upon completing each ratio, which may be critical for the expected neural effects of reinforced responding (e.g., McClure et al., 2007). Details for obtaining and verifying the content for each reward is described elsewhere (Perkins and Karelitz, 2013a, 2014). “Earnings” for each respective reward trial averaged 3.9 mins of music, 3.9 mins of video, and $0.75 for money, or just under 8 reinforcers for each reward trial, showing all three were comparable in reinforcing efficacy (Honig and Staddon, 1977).
Importantly, subjects were told to work on the task only as long as they wanted the designated reward. They could stop responding at any point prior to the end of the 15-min task period and simply wait quietly or read the available magazines (which were intentionally of minimal interest so they would not serve as alternative reinforcers). As expected, all discontinued responding during each trial, showing that a maximal amount of responding to earn each reward was reached. Mean (SE) durations of responding per trial were 7.7±0.6 min for music, 8.0±0.5 min for video, 7.8±0.8 min for money, consistent with the comparable earnings for each (above), and 2.7±0.4 min for no reward.
2.4 Procedure
2.4.1 Study Sessions
Prospective participants attended an introductory session to provide written informed consent, verify eligibility, and learn the Applepicker task to become familiar with it. Three of the subsequent 2-hr experimental sessions followed overnight abstinence and differed only in the e-cigarette condition in effect: nicotine (36 mg) or placebo (0 mg) e-cigarettes presented under blind conditions, or no e-cigarette. A fourth session (occasionally labeled here “ad lib”) involved no overnight abstinence and use of subject’s preferred tobacco cigarette brand (unblinded). The purpose was to compare reinforcement enhancing effects of nicotine between acute intake from e-cigarettes after overnight abstinence and intake from intermittent tobacco smoking after no abstinence (i.e., their typical nicotine intake). The order of these e-cigarette conditions and one tobacco smoking condition across sessions was counter-balanced between subjects.
Upon arrival to each session, participants completed the MNWS nicotine withdrawal measure (Hughes and Hatsukami, 1986) and provided CO (Breathco CO monitor; Vitalograph, Lenexa, KS). CO criteria confirmed overnight (>12 hr) smoking abstinence in three sessions (CO≤ 10 ppm) and non-abstinence in one (CO>10 ppm), consistent with recommendations by the SRNT Subcommittee on Biochemical Verification (2002). Before each of the 4 task trials on the two e-cigarette sessions, participants self-administered the designated e-cigarette as described below. Trials lasted 25 mins each, to provide 5 mins for controlled intake of puffs, 2 mins to complete subjective responses, completion of the 15-min task period, and 3 mins for brief rest until the next trial. The MNWS withdrawal measure was again assessed at the end of each session to gauge differences due to intermittent puffs on the nicotine and placebo e-cigarettes, compared to no e-cigarette use. Participant compensation for completing the entire study was $200. This study protocol was approved by the University of Pittsburgh Institutional Review Board.
2.4.2 Puff Topography Control
Prior to each trial during the two e-cigarette sessions, subjects self-administered 10 puffs over 5 mins from either e-cigarette, one puff every 30 sec, similar to recent studies assessing acute e-cigarette effects (e.g., Vansickel and Eissenberg 2013; Dawkins and Corcoran 2014; Nides et al., 2014; Spindle et al., 2015). On the comparison non-abstinent tobacco smoking session, they took 6 puffs over 3 mins before each trial, again one every 30 sec, as in our prior research (Perkins and Karelitz, 2014). All puffing was done via portable Clinical Research Support System (“CReSS Pocket”), with an adapter (“E-Cig Adaptor 9.00 mm”) for use with e-cigarettes as necessary. Both were obtained from Borgwaldt KC, Inc. (Richmond VA). Also as in prior studies standardizing tobacco cigarette topography (Perkins et al., 2012), the precise timing and duration of each puff inhalation were guided by computer-presented instructions, with different puff hold durations for each product (4-sec for e-cigarettes, 2-sec for the tobacco cigarette).
2.4.3 Subjective Responses to E-Cigarette Nicotine
To gauge differences due to acute nicotine delivery via the two e-cigarettes, subjects completed 7 subjective responses immediately after finishing the first set of 10 puffs (e.g., “how much nicotine”, “liking”, and “taste”). These items were adapted from a measure of subjective responses to tobacco smoking (Westman et al., 1996), with each response indicated on a 0-100 visual analog scale (anchored by “not at all” and “extremely”).
2.5 Data Analyses
In analyses, conducted using IBM SPSS 22.0, the main dependent measure was reinforced behavior (number of task responses) for each reward, just one available per trial. Preliminary analyses found no effects of the 6 possible orders for the 3 rewards across trials (ignoring no reward), as well as of e-cigarette condition order across sessions. Thus, data for the three sessions involving overnight abstinence were collapsed across orders in the subsequent primary analyses of variance (ANOVAs) of these responses, which involved two within-subjects factors, e-cigarette condition (none, placebo, nicotine) and type of reward trial (4). A priori pairwise comparisons (Huitema, 1980) of responding were conducted for each reward, to test differences between the nicotine vs. placebo e-cigarette conditions (isolating acute nicotine effects per se) and between the placebo e-cigarette vs no e-cigarette conditions (isolating effects of e-cigarette use behavior per se). Effects of nicotine, but not e-cigarette use, were expected to be significant for music and video rewards, and not significant for monetary reward, based on prior studies of acute nicotine effects via tobacco smoking (e.g., Perkins et al., 2013a; Perkins and Karelitz, 2014). Similar analyses were conducted for withdrawal (change from baseline) and on the topography and trial 1 subjective responses to each e-cigarette condition (nicotine vs. placebo). Topography was missing for one participant during one trial, so N=27 for that analysis. No differences were significant due to the main or interaction effects of sex (all Fs<1), and so this between-subjects factor is not addressed further.
Finally, we conducted similar analyses of reinforced responding between the nicotine e-cigarette session versus the preferred brand tobacco smoking session (i.e., “ad lib”, or no abstinence), to explore how much the effects due to acute nicotine intake from an e-cigarette after overnight abstinence differed from the non-abstinent, satiated condition (see Perkins and Karelitz, 2013b). Also, we assessed differences between the smoking and no e-cigarette conditions to replicate prior findings on effects of smoking abstinence vs. non-abstinence on reinforced responding by type of available reward (Perkins and Karelitz, 2013a; Perkins et al., 2013).
3. RESULTS
3.1 Reinforcing value of rewards
The group’s mean (SE) responding is shown in Figure 1, by the three e-cigarette/abstinent conditions and the comparison smoking/non-abstinent condition, presented separately for each reward. Overall reinforced responding was significantly influenced by the main effect of reward type, F(3,81)=25.46, p<.001, as responding was similar for the 3 rewards and far more than for the no reward control trial (as expected). Total responding did not vary by e-cigarette condition, F(2,54)<1, but the e-cigarette x reward interaction was marginally significant, F(6,162)=1.91, p=.08. More importantly, in the a priori planned comparisons by each reward, reinforced responding for video reward increased by 181±60 after the nicotine vs placebo e-cigarette, t(27)=3.03, p<.005, with a Cohen’s d value for effect size of 0.46. The placebo e-cigarette vs. no e-cigarette conditions did not differ from each other, t(27)=0.16, p=.87. These comparisons indicated that nicotine intake per se, and not simply e-cigarette use behavior, enhanced reinforced responding for video reward.
Figure 1.
Mean+SEM number of responses for reinforcers, by reward type and e-cigarette (or smoking) condition (N=28). Responding for video reward, but not music, money, or no reward, was significantly increased by the nicotine vs. placebo e-cigarette (*** p<.005). Also shown for comparison is responding during the non-abstinent tobacco smoking session, in which † p<.05 for smoking vs. nicotine e-cigarette, and † p<.05 for smoking vs. no e-cigarette (and smoking vs. placebo e-cigarette).
No differences in reinforced responding due to e-cigarette condition were found for money, F(2,54)=0.33, p=.72, or no reward control, F(2,54)=0.09, p=.91, as shown in our studies of nicotine via cigarette smoking (Perkins et al., 2013a; Perkins and Karelitz, 2014). Unexpectedly, and contrary to those prior studies, responding for music reward also was not influenced by e-cigarette condition, F(2,54)=0.67, p=.94, including nicotine vs. placebo, t(27)=0.26, p=.80. We conducted exploratory binomial tests on the number of the 28 subjects who responded more for each of the three rewards due to the nicotine vs. placebo e-cigarettes, compared to those responding more for the no reward control condition (i.e., similar to the inactive lever control in animal studies; e.g., Donny et al., 2003; Caggiula et al., 2009). Relative to the proportion responding more for no reward due to nicotine (39%, or 11), the proportion responding more for music (64%, 18; p=.006) or for video reward (68%, 19; p=.002) was greater, but not so for money (50%, 15; p= .159). This post hoc observation suggests some similarity in the qualitative, if not necessarily quantitative, effects of nicotine e-cigarette use on responding for these sensory rewards.
3.2 Other responses to E-Cigarettes
3.2.1. Use and subjective responses
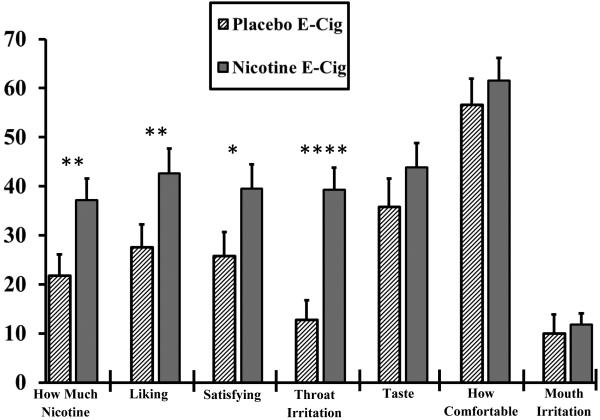
Topography was no different between the nicotine and placebo e-cigarettes, F(1,26)=1.23, p=.28, as volume from the 10 puffs per trial was 1034±78 vs. 1128±67 ml, respectively, very similar to volume per puff from the same pattern of e-cigarette use in other research (e.g., Spindle et al., 2015). Topography also did not differ due to reward type, F(3,78)=1.34, p=.27, ranging from 1033±61 for video to 1119±71 for no reward, or due to nicotine x reward interaction, F(3,78)=0.92, p=.44. In contrast, subjective responses differed between e-cigarettes, F(7,21)=9.92, p<.001. As shown in Fig 2, pairwise comparisons found greater ratings for the nicotine vs. placebo e-cigarette on how much nicotine, as well as on liking, satisfied, and throat irritation, but not on taste, comfortable, or mouth irritation.
Figure 2.
Mean+SEM subjective responses to the first trial (10 puffs) of exposure to the nicotine or placebo e-cigarette. * p<.05, ** p<.01, **** p<.001 between e-cigarettes.
3.2.2. Withdrawal
Change in withdrawal from baseline across e-cigarette conditions was also significantly affected, F(2,54)=6.92, p<.005. The baseline level after overnight abstinence, which did not differ by condition, dropped at post-trial 4 for the nicotine (33.5±3.9 to 20.1±3.5, respectively) and the placebo (30.1±4.3 to 25.3±3.7) e-cigarette, but not following no e-cigarette use (31.8±3.9 to 31.9±4.2), as expected.
3.3 Comparisons with Nicotine from Tobacco Smoking (Non-Abstinence)
Also displayed in Fig. 1 are the corresponding reinforced responses for each reward during the non-abstinent tobacco smoking condition (i.e., “ad lib” use of own brand). Compared to that in the nicotine e-cigarette condition, such responding was similar for video and for no reward, F(1,27)=1.42 and 1.48, respectively, both p>.20, but greater for music, F(1,27)=6.24, p<.02, and marginal for money, F(1,27)=4.22, p<.06. Other responses during the tobacco smoking condition were very consistent with our prior studies of reinforcement enhancing effects of nicotine via smoking (Perkins et al., 2013a, Perkins and Karelitz, 2014). Reinforced responses during smoking were significantly increased over both non-nicotine conditions, i.e., no e-cigarette and placebo e-cigarette, for music, F(1,27)=6.19 and 4.90, respectively, both p<.05, and for video, F(1,27)=6.88 and 7.45, both p<.05, but not for money, F(1,27)=2.23 and 2.85, both p>.10, or for no reward control, both F(1,27)<1. As expected, withdrawal was low at baseline and post-trial 4 for the smoking/non-abstinence condition (9.5±1.3 to 10.0±1.1).
4. DISCUSSION
Partly consistent with prior research (Perkins and Karelitz, 2014), reinforced responding for video reward was significantly enhanced by use of a nicotine e-cigarette, compared to a placebo e-cigarette or no e-cigarette use, which did not differ. Consequently, this reinforcement enhancing effect specific to sensory reward from video was due to acute nicotine intake per se from a non-tobacco nicotine product, and not from behavioral effects of simply using an e-cigarette. To our knowledge, this is the first demonstration of reinforcement enhancing effects of nicotine in humans via a source other than cigarette smoking. Nicotine’s effects on striatum may be relevant, as visual rewards may differentially activate striatum compared to others (Aharon et al., 2001; Lahnakoski et al., 2012), including monetary reward (Thut et al., 1997). Although speculative, enhancing reinforcement from video reward could partly help account for some of the growing appeal of nicotine e-cigarette use, especially if that use often occurs just before or during “leisure” activities, as is common with tobacco smoking (e.g., Hatsukami et al., 1990; Raiff et al., 2012; van Gucht et al., 2010). Such use may be even more likely with e-cigarettes than tobacco smoking, given fewer restrictions on use of the former in public and indoor locations, often reported as a reason for e-cigarette use (e.g., Adkison et al., 2013).
On the other hand, while this e-cigarette nicotine intake did not significantly increase responding for monetary reinforcement or the no reward control, as expected, it also did not increase responding for the music reward, as unexpected. The former results are very consistent with, but the latter is contrary to, our prior studies of nicotine from cigarette smoking, which consistently showed enhancement of music reinforcement (Perkins and Karelitz, 2013a, 2013b, 2014), as in the ad lib smoking session of the current study (see Fig. 1). Why music reward is enhanced by nicotine from tobacco smoking but perhaps not from e-cigarette use is uncertain. A more modest amount and/or slower uptake of nicotine from e-cigarettes vs smoking may explain this different effect of nicotine between products on enhancing music reinforcement. Regarding amount (i.e., dose), we have shown music reward is enhanced by nicotine from a full cigarette but not by intake from a half cigarette or less (Perkins and Karelitz, 2013b). In terms of uptake speed, we do not know how rapid nicotine is delivered by this specific e-cigarette brand, but recent research on comparable brands suggests quick rises in blood nicotine levels after the same puffing procedure used here, as noted earlier (e.g., Vansickel and Eissenberg, 2013; Hajek et al., 2015). Differential subjective responses immediately after the first exposure to the nicotine and placebo e-cigarettes, especially items that may be more closely tied to nicotine intake, are also consistent with rapid delivery (see Fig 2). Moreover, preclinical research has found that reinforcement enhancing effects of nicotine are not affected by speed of delivery (see Caggiula et al., 2009). Thus, smaller nicotine dosing via e-cigarette use here could be a simple explanation for lack of enhanced responding for music, although the amount was sufficient to enhance responding for video reward (as with the ad lib smoking). This observation could suggest differential dose-dependent relationships of nicotine with enhancement of sensory reward from video vs. music.
Also as in prior research (Perkins and Karelitz, 2014), we found virtually identical overall rates of responding across trials for the music, video, and monetary rewards, ruling out differential magnitude of reinforcing efficacy as an explanation for this specificity of nicotine e-cigarette effects on responses by reward type. Yet, the “video” reward here inextricably included some audio, along with visual stimulation (e.g., 30-sec clips of comedy routines), perhaps resulting in a more complex sensory experience than video that is silent, and these video rewards could be differentially enhanced by acute nicotine intake. Additional research on non-smoked nicotine is needed to replicate these findings and determine the mechanism by which nicotine does enhance music reinforcement via tobacco smoking but may not do so via e-cigarette use, even though nicotine from both products enhances video reinforcement.
Strengths of the study include the first direct test, to our knowledge, of the reinforcement enhancing effects of nicotine in humans with a non-smoked delivery method. Use of matching non-nicotine (placebo) e-cigarettes aided comparison due to nicotine intake per se, and careful control over topography of e-cigarettes was maintained. In terms of limitations, we do not have data on plasma nicotine levels after use of these e-cigarettes to confirm dose and speed of nicotine delivery, but subjective responses to each type immediately after the first 10 puffs was consistent with rapid intake of their expected differences in labeled nicotine content.
In any case, these findings extend prior results with tobacco smoking to partly confirm specificity of reinforcement enhancing effects of nicotine per se in humans, using non-smoked delivery. Combined with prior studies of nicotine via smoking, this study may suggest differential dose-response effects of acute nicotine on enhancing reinforcement between types of sensory reward (video vs. music). Confirmation would add acute dose amount or manner of nicotine delivery to the list of factors that can influence these effects, in addition to the sensory vs. non-sensory type of reward available for reinforcement of responding. Research should also expand testing to other types of nicotine-containing products that may enhance reinforcement in humans (e.g., lozenge, patch, gum) and to evaluating the conditions under which this occurs.
Highlights.
In humans, nicotine from tobacco enhances reinforcement from rewards unrelated to nicotine.
Nicotine via electronic cigarettes may also enhance reinforcement.
Nicotine from e-cigarette use enhances reinforcement from some but not all rewards.
Reinforcement enhancing effects of nicotine e-cigarette use may partly contribute to their growing popularity.
Acknowledgement
The authors thank Michael Eddy for help conducting this study.
Role of Funding Source
This research was supported by NIDA Grant DA35774 to KAP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No author has any potential conflicts of interest to report.
REFERENCES
- Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, Cummings KM, McNeill A, Thrasher JF, Hammond D, Fong GT. Electronic nicotine delivery systems: International tobacco control four-country survey. Am. J. Prev. Med. 2013;44:207–215. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual-IV. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Nat. Acad. Sci. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier M, Liu X, Chaudhri N, Sved A. The role of nicotine in smoking: a dual-reinforcement model. In: Bevins RA, Caggiula AR, editors. The Motivational Impact of Nicotine and its Role in Tobacco Use, Nebraska Symposium on Motivation; New York. Springer-Verlag; 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B, Dang M, Kim J, Talbot P. Nicotine concentrations in electronic cigarette refill and do-it-yourself fluids. Nicotine Tob. Res. 2015;17:134–141. doi: 10.1093/ntr/ntu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology. 2014;231:401–407. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Crowe E. Nicotine derived from the electronic cigarette improves time-based prospective memory in abstinent smokers. Psychopharmacology. 2013;227:377–384. doi: 10.1007/s00213-013-2983-2. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AC, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology. 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Etter J-F, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend. 2015;147:68–75. doi: 10.1016/j.drugalcdep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter J-F, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108:1671–1679. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- Fowler H. Implications of sensory reinforcement. In: Glaser R, editor. The Nature Of Reinforcement. Academic Press; New York: 1971. pp. 151–195. [Google Scholar]
- Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine Tob. Res. 2013;15:158–166. doi: 10.1093/ntr/nts103. [DOI] [PubMed] [Google Scholar]
- Hajek P, Goniewicz ML, Phillips A, Smith KM, West O, McRobbie J. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob. Res. 2015;17:175–179. doi: 10.1093/ntr/ntu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Morgan SR, Pickens RW, Champagne SE. Situational factors in cigarette smoking. Addict. Behav. 1990;15:1–12. doi: 10.1016/0306-4603(90)90002-f. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Honig WK, Staddon JER. Handbook Of Operant Behavior. Prentice-Hall; Englewood Cliffs NJ: 1977. [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Huitema B. Analysis Of Covariance And Alternatives. John Wiley & Sons; New York: 1980. [Google Scholar]
- Lahnakoski JM, Salmi J, Jaaskelainen IP, Lampinen J, Glerean E, Tikka P, Sams M. Stimulus-related independent component and voxel-wise analysis of human brain activity during free viewing of a feature film. Plos One. 2012;7 doi: 10.1371/journal.pone.0035215. Doi: 10.1371/journal.pone.0035215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J. Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Schaefer RMW, Winickoff JP, Klein JD. Trends in electronic cigarette use among U.S. adults: use is increasing in both smokers and nonsmokers. Nicotine Tob. Res. (in press) 2015 doi: 10.1093/ntr/ntu213. [DOI] [PubMed] [Google Scholar]
- Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am. J. Health Behav. 2014;38:265–274. doi: 10.5993/AJHB.38.2.12. [DOI] [PubMed] [Google Scholar]
- Norman WD, Jongerius JL. Apple Picker: Computer software for studying human responding on concurrent and multiple schedules. Behav. Res. Methods Instr. Comput. 1985;17:222–225. [Google Scholar]
- Perkins KA, Karelitz JL. Reinforcement enhancing effects of nicotine via smoking. Psychopharmacology. 2013a;223:479–486. doi: 10.1007/s00213-013-3054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Influence of reinforcer magnitude and nicotine amount on smoking’s acute reinforcement enhancing effects. Drug Alcohol Depend. 2013b;133:167–171. doi: 10.1016/j.drugalcdep.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. Sensory reinforcement-enhancing effects of nicotine via smoking. Exp. Clin. Psychopharmacol. 2014;22:511–516. doi: 10.1037/a0037823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob. Res. 2012;14:490–494. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC, Stratton E. Possible reinforcement enhancing effects of bupropion during initial smoking abstinence. Nicotine Tob. Res. 2013;15:1141–1145. doi: 10.1093/ntr/nts224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Responding maintained by primary reinforcing visual stimuli is increased by nicotine administration in rats. Behav. Proc. 2009;82:95–99. doi: 10.1016/j.beproc.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Jarvis BP, Rapoza D. Prevalence of video game use, cigarette smoking, and acceptability of a video-game based smoking cessation intervention among online adults. Nicotine Tob. Res. 2012;14:63–77. doi: 10.1093/ntr/nts079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Breland AB, Karaoghlanian NV, Eng M, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob. Res. 2015;17:142–149. doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Thut G, Schultz W, Roelcke U, Nienhusmeier M, Missimer J, Maguire RP, Leenders KL. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Van den Bergh O, Beckers T, Vansteenwagen D. Smoking behavior in context: where and when do people smoke? J. Behav. Ther. Exp. Psychiatry. 2010;41:172–177. doi: 10.1016/j.jbtep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine Tob. Res. 2013;15:267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol. Biochem. Behav. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]