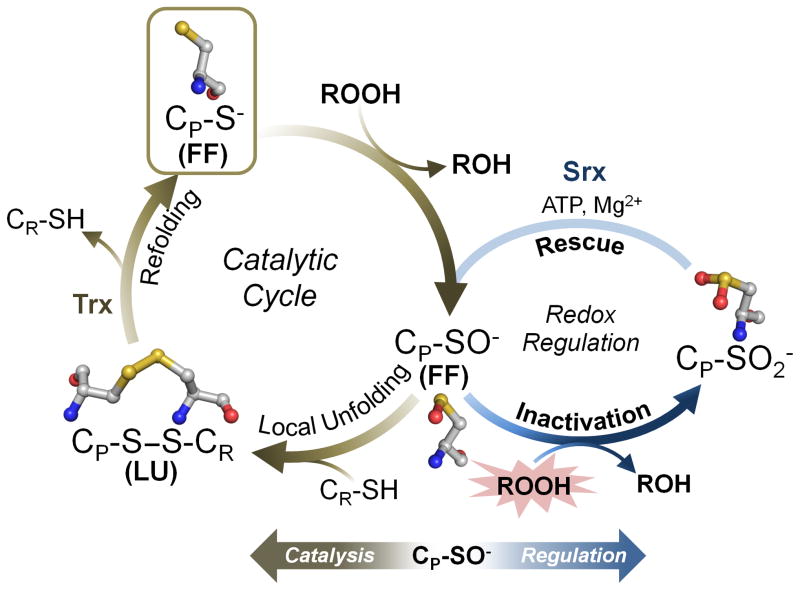
Figure 1.
The catalytic and regulatory cycles of 2-Cys peroxiredoxins (Prxs). Shown in brown is the normal Prx cycle with the structure of the peroxidatic Cys (CP) residue shown for each redox state (carbons are colored gray, nitrogens blue, oxygens red, and sulfurs yellow; hydrogens are not shown for simplicity). The CP thiolate (RS−) in the fully folded (FF) active site is first oxidized by the peroxide to form the sulfenic acid (R-SOH) or sulfenate (R-SO−) (computational approaches suggest stabilization of the CP as the sulfenate, but the true protonation state is as yet uncertain). This sulfenate, which must undergo a conformational change to become locally unfolded (LU), then forms a disulfide bond with the resolving Cys (CR) in 2-Cys Prxs. Reductive recycling by thioredoxin (Trx) or a Trx-like protein or domain (e.g., tryparedoxin or the N-terminal domain of bacterial AhpF) then restores the thiolate in the FF active site for another catalytic cycle. Shown in blue is the redox-linked regulatory cycle of predominantly eukaryotic Prxs, wherein the CP sulfenate becomes further oxidized, in the presence of high peroxide levels, to the inactive sulfinate (R-SO2−). In some organisms and Prx isoforms the active enzyme is restored by the ATP-dependent activity of sulfiredoxin (Srx).