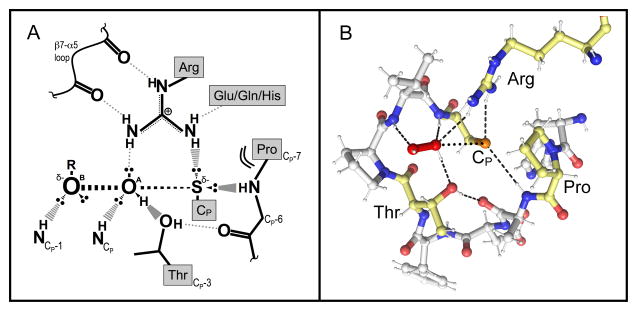
Figure 3.
The universally-conserved Prx active site. (A) Cartoon representation of the putative active-site transition state conformation. The stabilizing interactions between key atoms from the backbone and the four conserved residues, and with the ROOH substrate, are indicated. In the transition state, a bond is forming between the S atom of the CP and the OA of ROOH, and a bond is breaking between the OA and OB atoms of ROOH. The geometry of the active site appears nicely set up to stabilize the larger distance between the OA and OB atoms as the bond is broken. Based on a figure from Hall et al., 2010 [52]. (B) Shown is the Michaelis complex of a hydrogen peroxide-bound Prx (ApTpx, Protein Databank Identifier code 3A2V) with the four conserved residues of the active site motif highlighted in pale yellow and key active site hydrogen bonds noted with dashed lines. The interaction between Cp and the OA of H2O2 where the bond will form is depicted with dots.