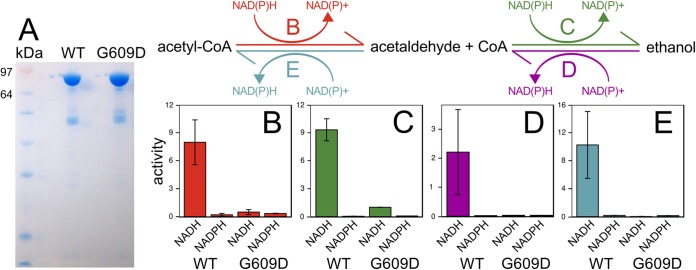
FIG 3.
Comparison of activities of purified Cphy3925 AdhE from wild-type and ET strains. (A) SDS-PAGE gel of purified Cphy3925 from the wild-type (WT) and ET (G609D) strains showing single bands of the expected 95-kDa molecular mass. (B to E) Reactions for the two-step, bidirectional interconversion of acetyl-CoA acetaldehyde and ethanol: reduction of 300 μM acetyl-CoA to acetaldehyde (red) (B), reduction of 18 mM acetaldehyde to ethanol (green) (C), oxidation of 2 M ethanol to acetaldehyde (purple) (D), and oxidation of 18 mM acetaldehyde to acetyl-CoA (blue) (E). Enzyme activities are shown in millimoles of NAD(P)H per micromole of enzyme per second measured using NADH(P)H or NAD(P)+ cofactors. Bar heights represent averages of duplicate activity measurements, and error bars represent 1 standard deviation.