Abstract
A revision of the classification of Geastrum sect. Geastrum is presented on the basis of an integrative taxonomic approach, which involves the study of morphological, molecular, ecological, and chorological data. Four DNA regions are analysed: the ITS and LSU nrDNA, rpb1, and atp6. Phylogenetic reconstructions include 95 ingroup samples and show five main clades, which are considered as five subsections, one of them proposed as new (G. subsect. Hungarica), and a total of 27 lineages recognizable at species level. Discriminant function analyses, ANOVAs and Tukey’s HSD tests on more than 500 basidiomata reveal the utility of several quantitative morphological characters for species delimitation. As a result of the combination of the different sources of taxonomic information, a revised taxonomy is presented and seven new species, viz., G. austrominimum, G. benitoi, G. britannicum, G. kuharii, G. meridionale, G. papinuttii, and G. thanatophilum, are proposed.
Keywords: chorology, ecology, Geastraceae, morphology, phylogenetics, species delimitation
INTRODUCTION
Taxonomy of earthstars (genus Geastrum) has been traditionally based on morphological traits of the basidiomata. Among the many studies about the taxonomy of the genus Geastrum, Sunhede’s monograph (Sunhede 1989) is by far the most comprehensive one. His taxonomic view and species circumscription are followed by most recent authors, sometimes with minor exceptions (e.g., Calonge 1998, Sarasini 2005, Jeppson 2013). However, a more complex scenario is coming to light with the inclusion of molecular phylogenetic data. For instance, Kasuya et al. (2012) found an unexpected phylogenetic diversity hidden under an a priori ‘single morphological species’ (G. triplex s.l.). The systematics of the whole genus Geastrum concerning infrageneric subdivisions is being elucidated thanks to DNAbased molecular data (Zamora et al. 2014), and the necessity of finding new morphological or chemical information sources to determine the phylogenetic and taxonomic boundaries has been also pointed out (Zamora et al. 2013, 2014).
Morphologically close taxa are an important source of taxonomic problems and disagreement in classificatory systems. So, it is necessary that adequate samples of specimens are available, in order to properly identify reliable differences and not only the extremes of the intraspecific variation. In these cases, multivariate analysis based on large sets of morphological data have proven to be useful for taxonomic purposes, by identifying morphologically homogeneous groups (Pimentel 1979, Valcárcel & Vargas 2010, Jiménez-Mejías et al. 2014). In particular, linear discriminant function analyses have been used in Botany for decades to identify useful characters that allow the distinction of different taxa (Henderson 2006), being the ‘Fisher’s Iris dataset’ the classical example of this type of analysis (Fisher 1936). However, few studies in Mycology have performed discriminant analyses for assessing morphological differences among species (Smith et al. 2004, Roca-Valiente 2013), despite they are very promising for morphology-based species identification.
It is known that different species delimitation approaches can produce highly deviant results, thus producing taxonomic conflicts that destabilise systematics. What is more, all methods have weaknesses and may fail in species delimitation (Schlick-Steiner et al. 2010, Carstens et al. 2013). Instead of fighting between two of the most used methods for species delimitation, i.e., phylogenetic and morphological species recognition, the combination of data from these approaches may result in more stable classifications accessible for a larger number of researchers. The sum of several taxonomic information sources has been sometimes called ‘integrative taxonomy’, a term firstly proposed by Dayrat (2005). However, Dayrat’s concept is rather strict and some of his guidelines are criticable, as Valdecasas et al. (2008) carefully explained. Even if it is not always easy to find balance among the different data, the results may reflect a more natural and accurate classification of the involved taxa (Ruiz-Sánchez & Sosa 2010, Medina et al. 2012, Edwards & Knowles 2014).
Among the 14 sections of Geastrum considered in Zamora et al. (2014), we decided to focus the present study on G. sect. Geastrum, which includes species with a glabrous endoperidial surface, coated by a mesoperidial layer made up by generative hyphae and crystalline matter; slender, ellipsoid to cylindrical basidia; double-layered mycelial layer encrusting debris; basidiospores with verrucose to baculate-pilate ornamentation; and rhizomorphs with mostly calcium oxalate dihydrate (COD) bipyramidal crystals, sometimes mixed with calcium oxalate monohydrate (COM) horn-like ones (G. ovalisporum, G. parvistriatum, G. pectinatum, G. striatum), but rarely only horn-like (G. coronatum) (Zamora et al. 2013). This section was subdivided into four subsections, distinguishable by some macromorphological traits (mainly the peristome type and the mesoperidium structure) as well as features of the basidiospores. Finally, G. hungaricum was indicated by Zamora et al. (2014) to possibly belong to this section, based on morphology and molecular data of Jeppson et al. (2013), but it was not definitely included awaiting further data.
Some of these species are considered to be well-known and apparently widespread (e.g., G. coronatum, G. minimum, G. pectinatum, G. quadrifidum, G. striatum), while some others are still undescribed. Paying special care to the literature, numerous taxonomic problems arise around different species included in G. sect. Geastrum. The so-called G. minimum is normally treated as a single, rather variable species, mostly small-sized and often showing quite big crystals of calcium oxalate on the endoperidial surface. Under this broad concept many names have been included as synonyms, such as the South African G. calceum, the North American G. juniperinum, and the European G. marginatum, G. cesatii, G. granulosum, and G. minimum var. fumosicollum (Cunningham 1944, Bottomley 1948, Sunhede 1989). Zamora et al. (2014) showed at least two well-separated phylogenetic lineages under the name ‘G. minimum agg.’, as well as samples provisionally determined as G. calceum.
Geastrum pectinatum is another widespread taxon that may involve more than one species. Palmer (1959) studied the type specimens of G. plicatum and G. tenuipes, concluding that both can be included under the variation of G. pectinatum, and Sunhede (1989), based on that study and on the protologues of these species, agreed with the synonymy. Geastrum biplicatum and G. calyculatum are also often considered synonyms (Cunningham 1944, Bottomley 1948). Zamora et al. (2014) showed a single clade containing samples from various worldwide locations, but with a considerable variation in sequence data.
The South European and recently proposed G. parvistriatum (Zamora & Calonge 2007) has been carefully compared with other morphologically close European taxa, particularly to G. striatum, and the differences of both species are clearly established by both morphological and molecular phylogenetic data (Jeppson et al. 2013; Zamora et al. 2014). However, Zamora et al. (2014) included sequences of the Argentinian G. glaucescens, a species that seems to be phylogenetically very close to G. parvistriatum. Kuhar et al. (2013) provided a rather detailed description of G. glaucescens and pointed out the morphological similarities with G. parvistriatum, concluding that they are different species despite their close relationship.
There are other minor taxonomic problems in this group. For instance, G. arenarium, originally described from North America, has been considered a rather widespread taxon, having been recorded from Australia (Cunningham 1944), South Africa (Bottomley 1948), South Europe (Calonge & Zamora 2003), and South America (Kuhar et al. 2013). However, the iconography and descriptions of the South European specimens are somewhat deviating because of the exoperidial rays with a less marked hygrometry (Calonge & Zamora 2003, Jeppson 2013). Finally, G. striatum is often treated as a very well-defined taxon due to its unique ring-like apophysis (Sunhede 1989), but some extra-European records are based on specimens with not so well-developed apophyses (e.g., Ochoa & Moreno 2006), and may represent different species.
Thus, the main goal of this study is to clarify the systematics of Geastrum sect. Geastrum using both molecular and morphological approaches, also taking into account other sources of information as well, such as chorology and ecology. This integrative approach lead us to describe the new taxa found during this study, determining whether the different phylogenetic clades obtained through molecular data can be distinguished on account of their morphological characteristics.
MATERIAL AND METHODS
Study group and sampling strategy
The study group comprises all species and species complexes included by Zamora et al. (2014) as members of Geastrum sect. Geastrum, i.e., G. arenarium s.l., G. calceum s.l., G. coronatum s.l., G. glaucescens s.l., G. leptospermum, G. minimum s.l., G. ovalisporum, G. parvistriatum, G. pectinatum s.l., G. quadrifidum, G. smithii s.l., and G. striatum. Geastrum hungaricum was also added to test whether it nests or not in this section, since previous phylogenies have shown this species more or less closely related to some G. sect. Geastrum species (Jeppson et al. 2013), and also some morphological traits suggested it belonging to sect. Geastrum (Zamora et al. 2014). Finally, additional herbarium specimens not included in Zamora et al. (2014), but with morphological characters that match to those that define the sect. Geastrum, were added. The studied specimens came from the following herbaria: AH, B, BAFC, BPI, CANB, CORD, CUP, K, LPS, MA-Fungi, MEL, MICH, PRM, S, UFRN, UPS, and the private herbaria of M.Á. Ribes, S. Sunhede, J.C. Zamora, Asociación Vallisoletana de Micología (AVM), and Sociedad Micológica Errotari (ERRO).
Ninety five ingroup specimens were included in our molecular analyses (Table 1). A special effort was made in sampling specimens of G. minimum s.l. and G. pectinatum s.l., since Zamora et al. (2014) revealed various well-defined phylogenetic lineages for each of these taxa. Two specimens of G. fornicatum were used as outgroup based on Zamora et al. (2014). To minimize the inclusion of missing data, molecular data of specimens present in the GenBank database not generated by us were only included for certain groups (especially the G. minimum group and the G. pectinatum group), when either more than half of the DNA regions used were available for the same specimen, or when we lack newly generated sequences of a particular species.
Table 1.
Summary of specimens included in molecular analyses, for which geographical origin, herbarium vouchers, and GenBank accession numbers for each DNA region are provided. New sequences generated in this study are marked in bold.
| Taxon | Country and state/province | Herbarium voucher | GenBank accession numbers | |||
|---|---|---|---|---|---|---|
| ITS | LSU | rpb1 | atp6 | |||
| G. arenarium | Argentina, La Rioja | MA-Fungi 83760 | KF988351 | KF988471 | KF988606 | KF988741 |
| G. austrominimum | Australia, New South Wales | CANB 748741 | - | KP687529 | KP687531 | KP687572 |
| Australia, New South Wales | MEL 2276089 | KP687490 | KP687451 | KP687532 | KP687573 | |
| Australia, Victoria | MEL 2292062 | KP687491 | KP687452 | KP687533 | KP687574 | |
| Australia, Victoria | MEL 2358014 | KP687492 | KP687453 | KP687534 | KP687575 | |
| Australia, Victoria | MEL 2358047 | KP687493 | KP687454 | KP687535 | KP687576 | |
| G. benitoi | Spain, Madrid | MA-Fungi 68191 | KF988350 | KF988469 | KF988604 | KF988739 |
| Spain, Madrid | MA-Fungi 87324 | KP687494 | KP687455 | KP687536 | KP687577 | |
| Spain, Madrid | Zamora 76 | KF988338 | KF988470 | KF988605 | KF988740 | |
| G. cf. biplicatum | Japan, Mie | TNS Sakamoto 182 | JN845113 | JN845231 | - | JN845355 |
| Japan, Shizuoka | TNS Sakamoto 191 | JN845114 | JN845232 | - | JN845356 | |
| Japan, Aomori | TNS TKG-GE-91003 | JN845110 | JN845228 | - | JN845352 | |
| Japan, Shizuoka | TNS TKG-GE-91102 | JN845115 | JN845233 | - | JN845357 | |
| G. britannicum | England, Hampshire | K(M)60288 | EU784242 | - | - | - |
| England, Norfolk | K(M)79617 | EU784243 | - | - | - | |
| England, Norfolk | K(M)99914 | EU784244 | - | - | - | |
| G. cf. calceum1 | Argentina, Tucumán | MA-Fungi 83761 | KF988341 | KF988478 | KF988613 | - |
| G. cf. calceum2 | Brazil, Rio Grande do Norte | UFRN-Fungos 723 | KF988340 | KF988477 | KF988612 | KF988747 |
| G. coronatum | Hungary, Nagyerdö Csokás | PRM 842868 | KP687495 | KP687456 | KP687537 | - |
| Spain, Madrid | Zamora 181 | KP687496 | KP687457 | KP687538 | KP687578 | |
| Spain, Madrid | Zamora 266 | KF988361 | KF988483 | KF988618 | KF988753 | |
| Spain, Sweden | Zamora 522 | KF988362 | KF988484 | KF988619 | KF988754 | |
| USA, Arizona | MICH 28567 | KF988363 | KF988485 | KF988620 | KF988755 | |
| G. fornicatum | Spain, Lérida | MA-Fungi 30749 | KF988375 | KF988497 | KF988632 | KF988767 |
| Spain, Valladolid | Zamora 255 | KF988374 | KF988496 | KF988631 | KF988766 | |
| G. glaucescens | Argentina, La Rioja | MA-Fungi 83762 | KF988378 | KF988500 | KF988635 | KF988770 |
| Argentina, Catamarca | MA-Fungi 83763 | KF988379 | KF988501 | KF988636 | KF988771 | |
| G. granulosum | Russia, Rostov | K(M)154623 | JN845105 | JN845223 | - | JN845347 |
| Spain, Madrid | MA-Fungi 69175 | KP687497 | KP687458 | KP687539 | KP687579 | |
| USA, Arizona | MICH 28119a | KP687498 | KP687459 | KP687540 | KP687580 | |
| USA, Arizona | MICH 28210 | KP687499 | KP687460 | KP687541 | KP687581 | |
| USA, Wisconsin | MICH 72010 | KF988402 | KF988530 | KF988665 | KF988797 | |
| Sweden, Öland | Sunhede 7746 | KF988401 | KF988529 | KF988664 | KF988796 | |
| Spain, Madrid | Zamora 191 | KF988400 | KF988528 | KF988663 | KF988795 | |
| G. hungaricum | Hungary | GB MJ8915 | KC581964 | KC581964 | - | - |
| Slovakia | GB MJ9317 | KC581963 | KC581963 | - | - | |
| Czech Republic, Reporyje | Sunhede 5993 | KP687500 | KP687461 | KP687542 | KP687582 | |
| Spain, Toledo | Zamora 611 | KP687501 | KP687462 | KP687543 | KP687583 | |
| G. kuharii | Argentina, Buenos Aires | MA-Fungi 83795 | KF988463 | KF988598 | KF988733 | KF988864 |
| Argentina, Entre Ríos | MA-Fungi 86913 | KP687502 | KP687463 | KP687544 | KP687584 | |
| Argentina, Buenos Aires | MA-Fungi 86914 | KP687503 | KP687464 | KP687545 | KP687585 | |
| G. marginatum | Spain, Canary Islands | ERRO 2012112609 | KP687504 | KP687465 | KP687546 | KP687586 |
| Spain, Madrid | MA-Fungi 31530 | KF988404 | KF988532 | KF988667 | KF988799 | |
| Spain, Jaén | MA-Fungi 32395 | KP687505 | KP687466 | KP687547 | KP687587 | |
| Spain, Madrid | MA-Fungi 48129 | KP687506 | KP687467 | KP687548 | KP687588 | |
| Sweden, Gotland | MA-Fungi 86669 | KF988405 | KF988533 | KF988668 | KF988800 | |
| USA, Arizona | MICH 28119b | KF988403 | KF988531 | KF988666 | KF988798 | |
| Czech Republic, Bohemia | PRM 842884 | KP687507 | KP687468 | KP687549 | - | |
| G. meridionale | Spain, Valladolid | AVM 2708 | KP687508 | KP687469 | KP687550 | KP687589 |
| Spain, Mallorca | MA-Fungi 20615 | KP687509 | KP687470 | KP687551 | KP687590 | |
| Portugal, Estremadura | MA-Fungi 31164 | KP687510 | KP687471 | KP687552 | KP687591 | |
| Spain, Córdoba | MA-Fungi 55189 | KP687528 | KP687472 | KP687553 | KP687592 | |
| Spain, Cádiz | MA-Fungi 59644 | KP687511 | KP687473 | KP687554 | KP687593 | |
| Spain, Madrid | MA-Fungi 87325 | KF988412 | KF988540 | KF988675 | KF988808 | |
| Spain, Canary Islands | Ribes 301207-43 | KP687512 | KP687474 | KP687555 | KP687594 | |
| Spain, Guadalajara | Zamora 276 | KP687513 | KP687475 | KP687556 | KP687595 | |
| G. ovalisporum | Bolivia, Concepción | MA-Fungi 47184 | KF988411 | KF988539 | KF988674 | KF988805 |
| Argentina, Salta | MA-Fungi 86670 | - | KP687476 | KP687557 | - | |
| Brazil, Rio Grande do Norte | UFRN-Fungos 229 | KP687514 | - | - | - | |
| G. papinuttii | Argentina, Santiago del Estero | MA-Fungi 83764 | KF988380 | KF988502 | KF988637 | KF988772 |
| Argentina, Santiago del Estero | MA-Fungi 86912 | KP687515 | KP687477 | KP687558 | KP687596 | |
| G. parvistriatum | Spain, Madrid | MA-Fungi 69583 | JN943160 | JN939560 | JN991291 | KF988806 |
| Spain, Madrid | Zamora 272 | JN943162 | JN939572 | JN991283 | KF988807 | |
| Spain, Madrid | Zamora 285 | JN943161 | JN939571 | JN991282 | KP687597 | |
| G. pectinatum | Spain, Lugo | MA-Fungi 28156 | KP687516 | KP687478 | KP687559 | KP687598 |
| Belgium | MA-Fungi 43295 | KP687517 | KP687479 | KP687560 | - | |
| Spain, Burgos | MA-Fungi 75533 | KP687518 | KP687480 | KP687561 | - | |
| Japan, Ibaraki | TNS TKG-GE-70901 | JN845111 | JN845229 | - | JN845353 | |
| Japan, Ibaraki | TNS TKG-GE-91201 | JN845112 | JN845230 | - | JN845354 | |
| Sweden, Uppland | UPS F-149330 | KP687519 | KP687481 | KP687562 | KP687599 | |
| Sweden, Gotland | UPS F-560803 | KF988413 | KF988541 | KF988676 | - | |
| Spain, Huesca | Zamora 290 | KP687520 | KP687482 | KP687563 | KP687600 | |
| Spain, Gerona | Zamora 292 | KP687521 | KP687483 | KP687564 | KP687601 | |
| G. cf. plicatum | Argentina, Buenos Aires | MA-Fungi 83774 | KF988415 | KF988543 | KF988678 | KF988810 |
| Argentina, Entre Ríos | MA-Fungi 87322 | KP687522 | KP687484 | KP687565 | KP687602 | |
| Tanzania, Iringa | UPS F-09935 | KF988414 | KF988542 | KF988677 | KF988809 | |
| G. quadrifidum | Sweden, Uppland | MA-Fungi 86671 | KF988422 | KF988550 | KF988685 | KF988817 |
| USA, Colorado | MICH 72512 | KF988423 | KF988551 | KF988686 | KF988818 | |
| Sweden, Sodermanland | S F-45993 | JN845119 | JN845237 | - | JN845361 | |
| Spain, Orense | Zamora 139 | KP687523 | KP687485 | KP687566 | KP687603 | |
| Spain, Huesca | Zamora 170 | KF988421 | KF988549 | KF988684 | KF988816 | |
| Spain, Cuenca | Zamora 300 | KP687524 | KP687486 | KP687567 | KP687604 | |
| G. smithii | Argentina, Córdoba | MA-Fungi 83783 | KF988442 | KF988575 | KF988710 | KF988841 |
| G. aff. smithii | Australia, New South Wales | CANB 748746 | KP687525 | KP687487 | KP687568 | KP687605 |
| G. striatum | Sweden, Uppland | MA-Fungi 86672 | KF988443 | KF988577 | KF988712 | KF988843 |
| Sweden, Narke | S F-46074 | JN845116 | JN845233 | - | JN845358 | |
| Sweden, Uppland | S F-74732 | JN845117 | JN845234 | - | JN845359 | |
| Spain, Madrid | Zamora 242 | JN943163 | JN939559 | JN991290 | KP687606 | |
| Spain, Madrid | Zamora 251 | JN943165 | JN939558 | JN991289 | KP687607 | |
| Spain, Valladolid | Zamora 257 | JN943164 | JN939557 | JN991288 | KF988842 | |
| G. aff. striatum | Mexico, Baja California | AH 18521 | - | KP687530 | KP687569 | KP687608 |
| G. tenuipes | Australia, Australian Capital Territory | CANB 738350 | KP687526 | KP687488 | KP687570 | KP687609 |
| Australia, Australian Capital Territory | CANB 775658 | KP687527 | KP687489 | KP687571 | KP687610 | |
| Australia, Victoria | MEL 2096557 | - | DQ218602 | - | DQ218889 | |
| G. thanatophilum | USA, Wisconsin | MICH 72012 | KF988364 | KF988486 | KF988621 | KF988756 |
| USA, Wisconsin | MICH 72014 | KF988365 | KF988487 | KF988622 | KF988757 | |
| Geastrum sp. | Japan, Aomori | TNS TKG-GE-91002 | JN845118 | JN845236 | - | JN845360 |
A total of 565 mature basidiomata were measured for morphometric analyses, and additional specimens not included in these analyses were examined to properly describe the proposed new species. Complete descriptions are provided in addition to the diagnosis for all new species, and only synoptic descriptions with the most relevant or diagnostic characters that define each taxon were included for the already described species. Those synoptic descriptions are based on both the literature (then references are provided) and newly observed data. Terminology mostly followed Sunhede (1989).
Due to the high amount of revised herbarium collections, for most taxa we only cite specimens used for molecular or morphological analyses. Specimens that significantly contributed to the intraspecific variation are also mentioned. In the case of the new species, all examined specimens are cited.
Ecological data were taken from the literature, herbarium labels, and our own observed data from newly collected specimens. Terminology of biomes and ecozones follows Olson et al. (2001).
Molecular analyses
Methodology concerning molecular analyses followed Zamora et al. (2014) and therefore it is summarized next.
The following DNA regions were studied: ITS (including ITS1, 5.8S, and ITS2) and 28S (LSU) nrDNA, rpb1, and atp6. Primers used for PCR amplification of the target fragments were: ITS1F (Gardes & Bruns 1993) and ITS4 (White et al. 1990) for the ITS region; LR0R (Cubeta et al. 1991) and LR5 (Vilgalys & Hester 1990) for LSU; gRPB1A (Stiller & Hall 1997)/fRPB1C (Matheny et al. 2002) and/or RPB1GEA-1F/RPB1GEA-2r (Zamora et al. 2014) for rpb1; and atp6-1, atp6-2, atp6-3 (Kretzer & Bruns 1999), ATP6GEA-ir, and ATPGEA-iF (Zamora et al. 2014) for atp6. Sometimes, ITS and LSU were amplified together, and then the primers used were ITS1F and LR5. PCR cycling parameters follow Martín & Winka (2000) for ITS, Zamora et al. (2014) for LSU (alone or together with ITS) and rpb1, and Kretzer & Bruns (1999) for atp6. PCR products were purified with ExoSap-IT® (VWR, Spain) or using the QIAquick® Gel Extraction Kit (QIAGEN, Germany). Sequencing was performed by Macrogen (The Netherlands).
DNA sequences were edited with Sequencher 4.1.4 (Gene Codes, USA), primarily aligned using the FFT-NS-i strategy of MAFFT (Katoh et al. 2002), and manually adjusted with BioEdit v. 7.0 (Hall 1999), except the atp6 region that was directly aligned with BioEdit reversely transcribed to protein format. Ambiguously aligned parts of ITS were removed with Gblocks v. 0.91b (Castresana 2000), keeping default settings but allowing all gap positions when not ambiguous. The remaining indels were coded with FastGap v. 1.2 (Borchsenius 2007), using the simple indel coding method of Simmons & Ochoterena (2000), in a separate binary data subset. Datasets are available in TreeBASE (TB2:S15972).
Maximum likelihood (ML) and Bayesian inference (BI) approaches were used for phylogenetic reconstructions. Conflicts among datasets were detected performing maximum parsimony bootstrap analyses of each region and identifying if a significantly supported clade (bootstrap value ≥ 70 %, Hillis & Bull 1993) from one DNA region is contradicted by another significantly supported clade from other regions. These preliminary bootstrap analyses were performed using the ‘fast’ stepwise-addition bootstrap implemented in PAUP* v. 4.0b10 (Swofford 2003), with 1 000 non-parametric bootstrap replicates (Felsenstein 1985). Datasets were concatenated when no conflict was detected.
ML analysis was done in GARLI v. 2.0 (Zwickl 2006), using the following partitions: ITS1, 5.8S, ITS2, LSU, rpb1, atp6, and coded indels. The GTR+I+Γ model was used for each DNA subset, and the Mk model for the binary subset (Lewis 2001). The analysis was repeated twice starting from random trees. For assessing branch supports, 1 000 non-parametric bootstrap replicates were performed under the thorough bootstrap option of RAxML v. 7.4.2 (Stamatakis 2006), using the mentioned partitions and default settings of searching parameters.
Bayesian inference was performed using the Metropolis Coupled Markov Chain Monte Carlo (MC3) strategy implemented in MrBayes v. 3.2.2 (Ronquist et al. 2012). DNA evolution models were selected with jModelTest v. 2.1 (Darriba et al. 2012), using the Akaike Information Criterion (AIC). The F81 model was used for the binary subset of coded indels. Four parallel runs were executed, each one starting with a random tree, with 6 chains, and length preset to 107 generations, sampling every 100th tree. The analysis was automatically stopped when the average standard deviation across runs dropped below 0.005. Convergence was additionally assessed using Tracer v. 1.5 (Rambaut et al. 2013), by checking that the values of effective sample size (ESS) for each parameter were ≥ 200, AWTY (Nylander et al. 2008) was used to discard lack of convergence by visual inspection of the plots obtained from the sampled trees. The first 25 % of the analysis was discarded as burn-in, and the 50 % majority-rule tree with branch lengths and posterior probabilities (pp) was calculated from the remaining trees. In a preliminary analysis, similar or even more severe problems noted by Zamora et al. (2014) concerning convergence and overestimation of branch lengths (Brown et al. 2010, Marshall 2010) were detected in the present study. However, the selection of an appropriate exponential prior (1/λ) for obtaining reasonable branch length estimates is often not obvious and several trials may be required (Ekman & Blaalid 2011). To avoid this, new and less informative priors have been implemented in MrBayes v. 3.2.2 (Rannala et al. 2012, Zhang et al. 2012). We used a uniform compound Dirichlet prior ‘brlenspr = unconstrained : gammadir (1,1,1,1)’, obtaining rather reasonable branch length estimates, and therefore this last analysis is the one that will be shown and discussed.
Relative strength of branch support values follows the scale of Lutzoni et al. (2004). Phylogenetic trees were drawn using FigTree v. 1.3 (Rambaut 2007).
Morphological analyses
General methodology for collecting morphological data followed Sunhede (1989), Calonge (1998), and Zamora et al. (2013). Macromorphological characters were measured in dried basidiomata. Micromorphological characters were measured in 5 % KOH solution or in Hoyer’s medium. Basidiospore measurements included the ornamentation following Sunhede (1989). Samples for scanning electron microscopy (SEM) were air dried, coated with pure gold in a Balzers SCD 004 sputter coater, and observed with a Hitachi S-3000N SEM. In some cases, fragments of the endoperidium were previously washed with absolute ethanol to remove the excess of basidiospores. For old collections and some type material, already detached fragments were used in order to avoid damaging of the specimens. Calcium oxalate crystals were assigned to COD or COM according to their habit (Frey-Wyssling 1981, Horner et al. 1995).
Continuous and discrete quantitative variables were measured on mature and dried herbarium basidiomata. Two morphometric approaches were used to test the utility of these characters in establishing differences among the studied taxa.
i) Most of the included species were more or less easily distinguished from the morphologically closest relatives according to several qualitative and quantitative morphological traits. For the newly proposed species, each quantitative character that may allow their distinction respect to the morphologically most similar taxa was tested through an ANOVA, considering p < 0.001 as significance value. When more than two taxa were involved, after detecting if there were significant differences with the ANOVA, the Tukey’s honestly significance difference (Tukey’s HSD) posthoc test was used to detect those means significantly different (p < 0.001) to each other. Measurements for each character were represented as boxplots. These analyses and graphics were done using R (R Development Core Team 2008). This approach was used for comparing G. benitoi with G. arenarium, G. britannicum with G. quadrifidum and G. leptospermum, and G. kuharii and G. thanatophilum with each other and with G. coronatum. When enough material was available, up to 50 basidiomata of each species were used to measure macromorphological characters, the largest mesoperidial crystals, and the broadest capillitial hyphae. For basidiospore features, 100 measurements were taken for G. arenarium, G. britannicum, G. kuharii, G. leptospermum, and G. thanatophilum, and 200 measurements for G. benitoi, G. coronatum, and G. quadrifidum, because the amount of material of these last three species was much higher.
ii) For three species groups that are particularly difficult to distinguish by morphology or have been consistently misinterpreted in the literature, we performed multivariate analyses on a wider dataset of quantitative morphological features. The three species groups so analysed were the ‘G. minimum group’ (G. austrominimum, G. calceum s.l., G. granulosum, and G. marginatum), the ‘G. glaucescens group’ (G. glaucescens, G. papinuttii, and G. parvistriatum), and the ‘G. pectinatum group’ (G. meridionale, G. pectinatum, G. plicatum, and G. tenuipes). Thus, multivariate analyses were used to detect and evaluate putative useful morphological characters for distinguishing some of the clades (putative taxa) found in the previous phylogenetic analyses. A priori assignation of specimens to particular species was done by using a combination of all available data sources (morphology, DNA sequences, ecology, and chorology). Geastrum calceum s.l. samples were treated as if they were a single species for morphological analyses due to the small sample size.
The suitability of the data for multivariate analyses was evaluated for each analysed dataset under the Kaiser-Meyer-Olkin measure of sampling adequacy (Kaiser 1974), calculated with the ‘rela’ package (Chajewski 2009) in R, and the Bartlett’s test of sphericity (Bartlett 1937), calculated with the ‘psych’ package (Revelle 2014), also in R. To avoid problems with multicollinearity, the correlation matrix between each variable was calculated in SPSS Statistics 22 (IBM Corp.), and variables with a regression coefficient ≥ 0.95 were identified. One variable of each pair with regression coefficient ≥ 0.95 was excluded in the subsequent analyses.
Multivariate analyses were performed in two steps.
a) The groups recognized in the phylogenetic analyses were tested using a linear discriminant function analysis (DFA). Samples were graphically represented as score plots, using the first two discriminant functions. The chi-square statistic of Wilks’ lambda test (Stevens 1996) was used to assess the discriminatory capabilities of the discriminant functions, i.e., if the functions differentiate the groups significantly. This set of multivariate analyses was performed in SPSS Statistics 22 (IBM Corp.). Finally, Cohen’s kappa and Z test of significance were calculated according to Titus et al. (1984), to evaluate whether there is agreement between the expected and the obtained classification of cases, and if such agreement may be a product of chance, having into account the sample sizes.
b) The six most discriminant characters of each group were represented as boxplots and analysed as in (i), i.e., first through an ANOVA and, when significant differences found, using the Tukey’s HSD posthoc test to identify what means were significantly different to others.
The following 13 morphological characters were measured: basidiospore diameter (BASDIA), ornamentation height (ORN), maximum ornamentation height (ORNMAX), maximum diameter of the capillitial hyphae (CAP), maximum diameter of isolated or twined, bipyramidal mesoperidial crystals of COD (CODCR), maximum diameter of mesoperidial crystalline aggregates of COM (COMCR, only for the G. minimum group), diameter of the exoperidium not forced in horizontal position (apparent exoperidial diameter, EXAP), diameter of the Exoperidium when extended or forced in horizontal position (real exoperidial diameter, EXEXT), number of exoperidial rays (RAYS), diameter of the endoperidial body (END), stalk length (STL), stalk width (STW, largest diameter in the middle part), and number of peristome folds (PER, only for the G. glaucescens and the G. pectinatum groups). In addition, the stalk height /stalk width ratio (STL /W) was calculated as an index of the robustness of the stalk. For basidiospore characteristics, 10–30 measurements were recorded per basidioma. Data used for DFAs are the mean values per basidioma rounded with a precision of 0.1 μm. For ANOVA analyses and boxplots representations, all measurements were considered to include the whole variation observed.
RESULTS
Molecular results
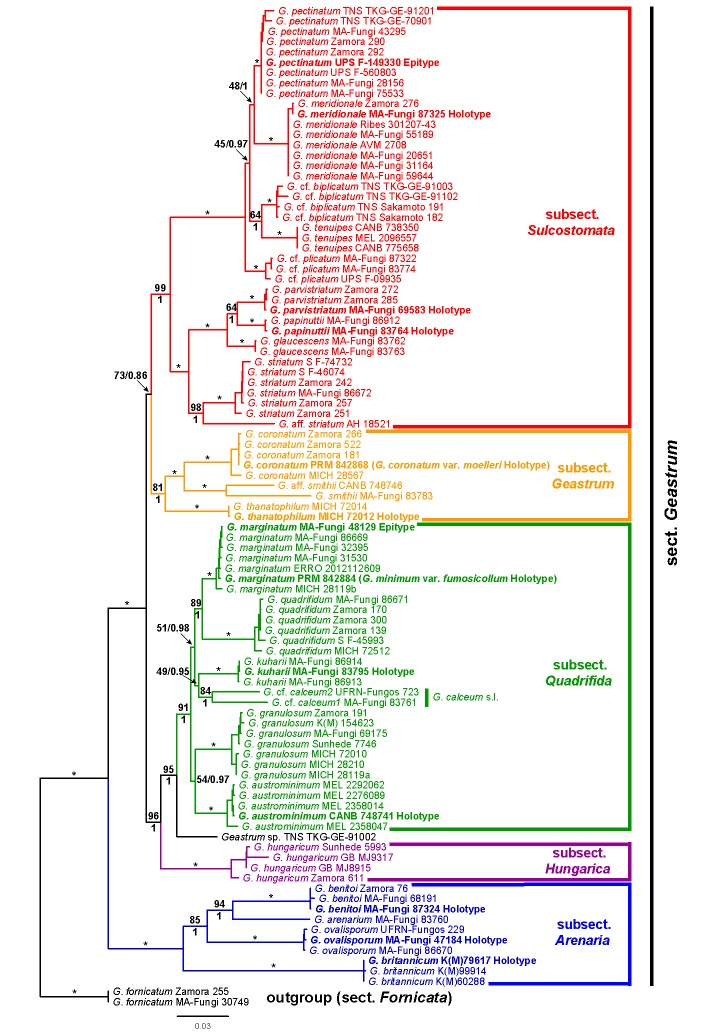
A total of 160 DNA sequences were newly generated in this study (Table 1). The concatenated matrix had 3 782 characters (235 ITS1, 155 5.8S, 197 ITS2, 987 LSU, 1 184 rpb1, 707 atp6, and 317 coded indels), of which 2 365 were constant and 1 417 variable. Maximum likelihood analyses recovered two trees with lnL1 = -22178.0906 (best) and lnL2 = -22178.0931, both with a similar topology. The ML tree of the first replicate (the one with the best likelihood score) is shown in Fig. 1.
Fig. 1.
Maximum likelihood phylogenetic tree of Geastrum sect. Geastrum. Numbers above branches indicate maximum likelihood bootstrap (bs) values, and numbers below branches indicate Bayesian posterior probability (pp) values. Asterisks (*) represent branches with bs = 100 % and pp = 1. Only support values above the species level are indicated. Type specimens are marked in bold.
The analysis with jModeltest yielded the following nucleotide substitution models: HKY+Γ for ITS1, K80 for 5.8S, GTR+Γ for ITS2, LSU, and rpb1, and GTR+I+Γ for atp6. Bayesian MC3 runs were automatically halted after 3 315 000 generations. Best likelihood states for each run were lnL1 = -22292.50, lnL2 = -22307.86, lnL3 = -22326.66, lnL4 = -22352.58. Potential Scale Reduction Factor values for model parameters were all between 1.000 and 1.002. The topology of the 50 % majority rule consensus tree is very similar to that of the ML tree, and then only pp values are indicated on branches of the ML tree (Fig. 1).
The ingroup (sect. Geastrum) of the ML tree (Fig. 1) is divided into five strongly supported (bs ≥ 81 %, pp = 1.00) main clades, considered as different subsections, which have been named from the base to the top of the tree as G. subsect. Arenaria, G. subsect. Hungarica, G. subsect. Quadrifida, G. subsect. Geastrum, and G. subsect. Sulcostomata. Geastrum subsect. Arenaria is composed by three strongly supported subclades (bs = 100 %, pp = 1.00), considered as three different species: G. britannicum, G. ovalisporum, and G. benitoi, plus one rather isolated specimen of G. arenarium. Geastrum subsect. Hungarica is composed by four specimens of a single species, G. hungaricum. Geastrum subsect. Quadrifida is formed by six strongly supported subclades (bs ≥ 84 %, pp = 1.00), distinguished as G. austrominimum, G. granulosum, G. calceum s.l., G. kuharii, G. quadrifidum, and G. marginatum. The G. calceum s.l. subclade is formed by two specimens, G. cf. calceum1 (MA-Fungi 83761) and G. cf. calceum2 (UFRN-Fungos 723), with notable differences in sequence data. Geastrum subsect. Geastrum groups two strongly supported subclades (bs = 100 %, pp = 1.00), namely G. coronatum and G. thanatophilum, plus two specimens, G. smithii (MA-Fungi 83783) and G. aff. smithii (CANB 748746), each one placed in wellseparated branches, with notable differences in sequence data. Geastrum subsect. Sulcostomata includes nine strongly supported subclades (bs = 100 %, pp = 1.00), recognized as the species G. striatum, G. glaucescens, G. papinuttii, G. parvistriatum, G. cf. plicatum, G. tenuipes, G. cf. biplicatum, G. meridionale, and G. pectinatum, plus one specimen placed in a well-separated branch under the name G. aff. striatum. Finally, the specimen TNS TKG-GE-91002 is not included in any subsection, and it is placed between G. subsect. Hungarica and G. subsect. Quadrifida.
Morphological results
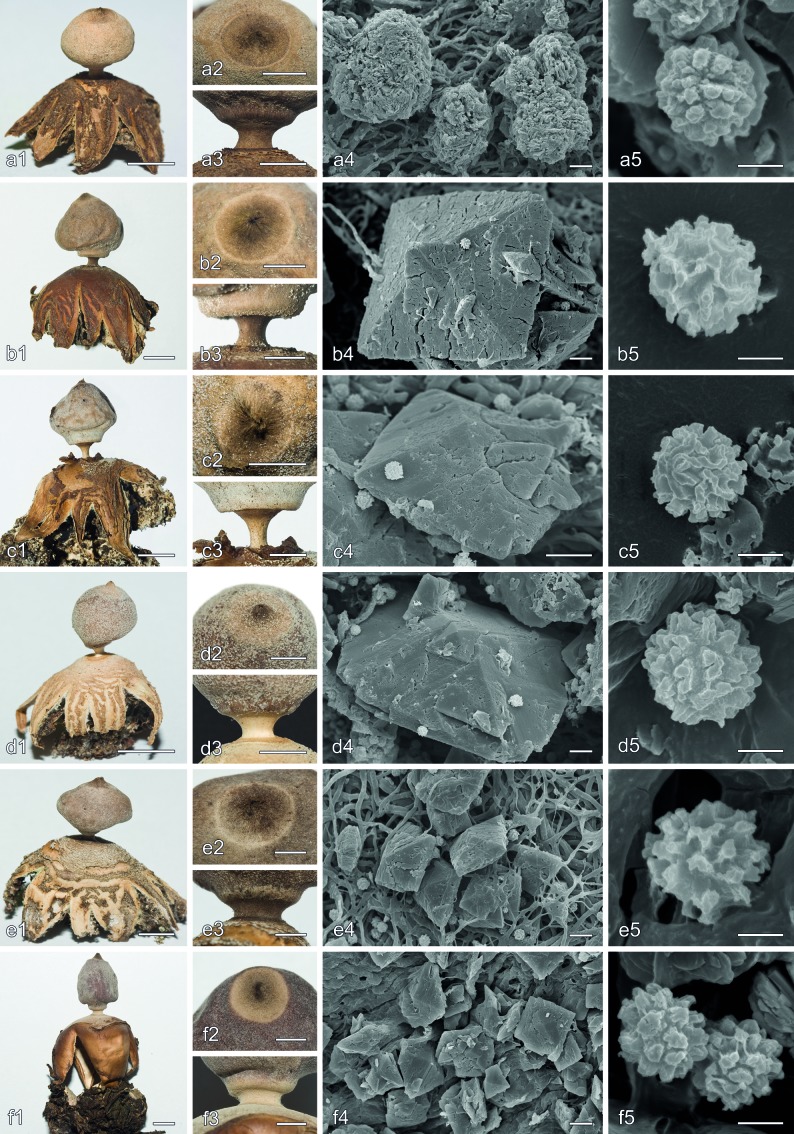
Scanning electron microscopy of the mesoperidial crystalline matter showed two well-differentiated morphological types, bipyramidal crystals of COD (Fig. 2a, b), and crystalline aggregates of COM (Fig. 2c). Bipyramidal crystals of COD can be isolated (Fig. 2a) to heavily twined (Fig. 2b), and were found in all species, although they were normally scarce in G. hungaricum. In G. benitoi, these bipyramidal crystals were often present in the form of bipyramidal prisms, with conspicuous faces (Fig. 9b4), easily visible under the light microscope, while in the other species mostly bipyramids or bipyramidal prisms with hardly distinguished or less conspicuous faces were present. Crystalline aggregates of COM were formed by numerous thin scales (Fig. 2c); although often rounded, they can be fused producing elongated forms. These crystalline aggregates have been only seen in species of Geastrum subsect. Hungarica and Geastrum subsect. Quadrifida, although they are normally rare or indistinct in G. calceum s.l. and G. quadrifidum. Geastrum granulosum and G. marginatum showed a broad variation of crystalline aggregates, that were normally scarce and not well-developed, but sometimes abundant and big. The mesoperidium of G. coronatum sometimes lacked any kind of crystalline matter.
Fig. 2.
Mesoperidial and rhizomorph calcium oxalate crystals. a–c. Mesoperidial crystals: a. G. marginatum (MA-Fungi 86669) single bipyramidal crystal of COD; b. G. kuharii (MA-Fungi 86913) twined bipyramidal crystals of COD; c. G. kuharii (MA-Fungi 86913) crystalline aggregate of COM scales. — d–f. Rhizomorph crystals: d. G. parvistriatum (Zamora 539) cystidioid cell covered by bipyramidal crystals of COD, some grouped in rose-like aggregates; e. G. coronatum (Zamora 484) arachnoid aggregate of thin horn-like COM crystals; f. G. leptospermum (lectotype) oblique prisms of COM grouped in stellate aggregates. — Scale bars = 10 μm.
Fig. 9.
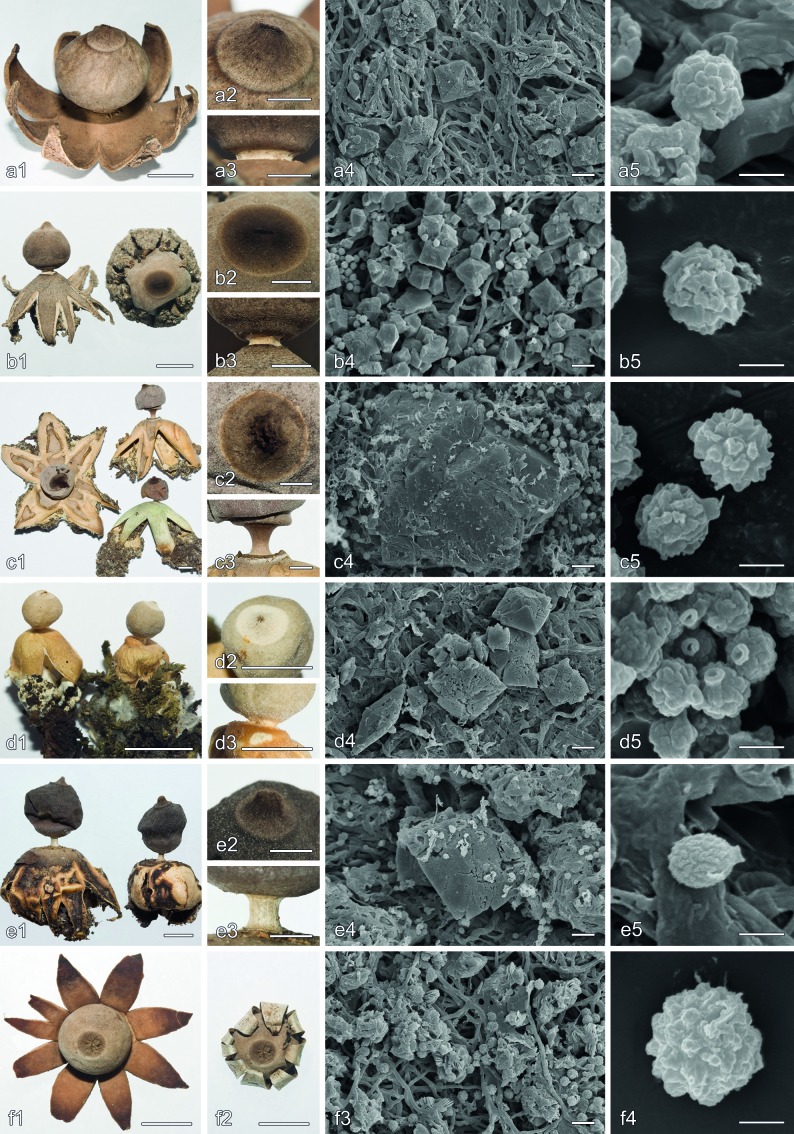
Morphological characters in Geastrum subsect. Arenaria and Geastrum subsect. Hungarica. a. G. arenarium (MA-Fungi 83760 and S (C.G. Lloyd 213)); b. G. benitoi (holotype); c. G. britannicum (holotype); d. G. leptospermum (lectotype); e. G. ovalisporum (holotype); f. G. hungaricum (Zamora 611). a1, b1, c1, d1, e1, f1, f2, basidiomata habit, f1 = f2 but wetted to show hygrometry of exoperidial rays; a2, b2, c2, d2, e2, detail of the peristome; a3, b3, c3, d3, e3, detail of the stalk and apophysis; a4, b4, c4, d4, e4, f3, mesoperidial crystalline matter on the endoperidial surface, a4, b4, c4, d4, e4 show bipyramidal crystals of COD, f3 shows crystalline aggregates of COM scales; a5, b5, c5, d5, e5, f4, basidiospores. — Scale bars: a1, b1, c1, d1, e1, f1, f2 = 5 mm; a2, b2, c2, d2, e2, a3, b3, c3, d3, e3 = 2 mm; a4, b4, c4, d4, e4, f3 = 10 μm; a5, b5, c5, d5, e5, f4 = 2 μm.
Rhizomorph crystal morphology largely agreed with the types recorded by Zamora et al. (2013). Bipyramidal crystals of COD, that were the most common type, were found in G. austrominimum, G. benitoi, G. granulosum, G. kuharii, G. meridionale, G. ovalisporum, G. papinuttii, G. parvistriatum, G. quadrifidum, G. striatum, and G. thanatophilum, forming rose-like aggregates or sometimes grouped on cystidioid-like cells (Fig. 2d). Irregular horn-like crystals of COM may be found also in G. meridionale, G. ovalisporum, G. parvistriatum, and G. striatum. In G. coronatum, thin horn-like crystals of COM, grouped in arachnoid structures, were the dominant type (Fig. 2e). Novel in this study is the presence of stellate aggregates of oblique prisms of COM in G. leptospermum rhizomorphs (Fig. 2f).
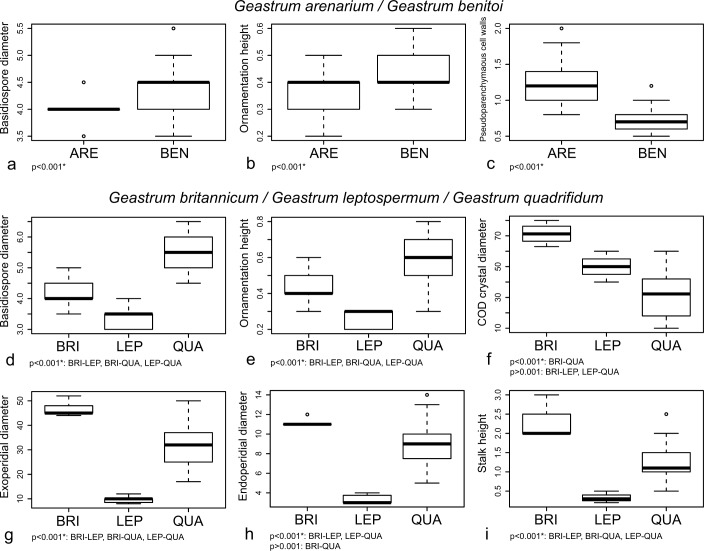
The three selected continuous variables for distinguishing between G. arenarium and G. benitoi showed significant differences (p < 0.001) (Fig. 3a, b, c). The walls of the pseudoparenchymatous layer cells were significantly thicker in G. arenarium, while basidiospores were significantly smaller and with a less marked ornamentation.
Fig. 3.
Boxplots representation of selected morphological characters for distinguishing new species in Geastrum subsect. Arenaria, and significance indices of ANOVA or Tukey’s HSD tests. a–c: G. arenarium and G. benitoi. a. Basidiospore diameter; b. ornamentation height; c. thickness of the pseudoparenchymatous cell walls. – d–i: G. britannicum, G. leptospermum, and G. quadrifidum. d. Basidiospore diameter; e. ornamentation height; f. maximum diameter of COD mesoperidial crystals; g. exoperidial diameter; h. endoperidial diameter; i. stalk height. Species names abbreviated as: ARE = G. arenarium, BEN = G. benitoi, BRI = G. britannicum, LEP = G. leptospermum, QUA = G. quadrifidum.
Geastrum britannicum, G. leptospermum, and G. quadrifidum also showed significant differences in all morphological characters selected (ANOVA p < 0.001) (Fig. 3d, e, f, g, h, i). Geastrum leptospermum showed the smallest and the least ornamented basidiospores, G. quadrifidum had the biggest and the most ornamented ones, and in G. britannicum basidiospores were intermediate between them. Tukey’s HSD test also showed significant differences in all possible comparisons among the three species (Fig. 3d, e). Geastrum britannicum showed the biggest mesoperidial crystals of COD, while G. quadrifidum had the smallest; G. leptospermum mesoperidial crystals showed an intermediate size. Tukey’s HSD test showed significant differences only between G. britannicum and G. quadrifidum (Fig. 3f). Geastrum britannicum was also the species with the largest basidiomata (exoperidial and endoperidial diameters, and stalk height), G. quadrifidum was somewhat smaller, and G. leptospermum was much smaller than both of them. Significant differences were found in Tukey’s HSD test for all possible comparisons of these characters except for the endoperidial diameter of G. britannicum and G. quadrifidum (Fig. 3g, h, i).
All the characters used for separating G. coronatum, G. kuharii, and G. thanatophilum showed significant differences (ANOVA p < 0.001) (Fig. 4). Geastrum coronatum had the largest basidiospores, G. kuharii the smallest, and in G. thanatophilum they were intermediate. Significant differences were found in Tukey’s HSD test for all possible comparisons (Fig. 4a). The capillitium of G. coronatum was significantly wider than in G. kuharii and G. thanatophilum, while significant differences were not found for these two last taxa (Fig. 4b). Geastrum kuharii and G. thanatophilum mesoperidial crystals of COD were rather similar between them and significantly bigger than those of G. coronatum (Fig. 4c). Basidiomata of G. coronatum were the biggest, represented by both the exoperidial and endoperidial diameters, and also showed the longest stalks, G. thanatophilum was the smallest and had the shortest stalks, and G. kuharii showed intermediate macromorphological characters between them; for these characters only comparisons between G. coronatum and G. thanatophilum showed significant differences (Fig. 4d, e, f).
Fig. 4.
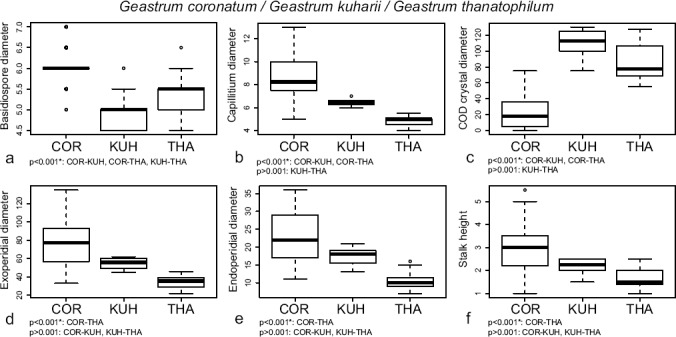
Boxplots representation of selected morphological characters for distinguishing morphologically similar new species in Geastrum subsect. Geastrum (G. thanatophilum) and Geastrum subsect. Quadrifida pro parte (G. kuharii), and significance indices of Tukey’s HSD tests. a. Basidiospore diameter; b. maximum diameter of the capillitial hyphae; c. maximum diameter of COD mesoperidial crystals; d. exoperidial diameter; e. endoperidial diameter; f. stalk height. Species names abbreviated as: COR = G. coronatum, KUH = G. kuharii, THA = G. thanatophilum.
The Kaiser-Meyer-Olkin measure of sampling adequacy was > 0.7, and the Bartlett’s test of sphericity was significant for all matrices used in multivariate analyses (Table 2). No variables showed regression coefficients ≥ 0.95 in correlation matrices, and therefore the whole datasets were used for DFAs. The chi-square test of Wilk’s lambda was significant for all discriminant functions (Table 2). In the G. minimum group dataset the percentage of correctly classified samples was more than 90 % for the four taxa studied. Cohen’s kappa value was > 0.85 for all species, but in G. calceum s.l. the Z test showed not significant results (Table 2). In the corresponding score plot, a low overlap is present among the different groups (Fig. 5a). In the G. glaucescens group dataset 100 % of G. papinuttii samples were correctly classified, but less than 80 % of the G. glaucescens and G. parvistriatum samples were correctly classified. Cohen’s kappa value was 1.00 for G. papinuttii, but < 0.65 for G. glaucescens and G. parvistriatum. The Z test was significant for all this values (Table 2). The score plot of this group showed a wide overlap between G. glaucescens and G. parvistriatum (Fig. 5b). In the G. pectinatum group dataset the percentage of correctly classified samples was 95 % for G. meridionale and 100 % for G. pectinatum, G. plicatum, and G. tenuipes. Cohen’s kappa value was > 0.90 for all species and the Z test was significant (Table 2). The score plot, representing the first two discriminant functions, showed that G. plicatum and G. tenuipes were clearly separated, while G. meridionale and G. pectinatum greatly overlap (Fig. 5c).
Table 2.
Results from DFAs indicating the number of basidiomata (N), Kaiser-Meyer-Olkin measure of sampling adequacy (KMO), Barlett’s test, Wilks’ lambda test, significance of chi-square statistic of Wilks’ lambda, percentage of correctly classified samples, Cohen’s kappa and significance of Z test (* means p < 0.001, ns means p > 0.001), and the six most discriminant variables selected for each group. AUS = G. austrominimum, CAL = G. calceum s.l., GRA = G. granulosum, MAR = G. marginatum, GLA = G. glaucescens, PAP = G. papinuttii, PAR = G. parvistriatum, MER = G. meridionale, PEC = G. pectinatum, PLI = G. plicatum, TEN = G. tenuipes.
| Dataset | N | KMO | Bartletts’s | Wilks’ λ | Sig. X2 Wilks’ λ | % correctly class. | Cohen’s K(sig) | Selected variables |
|---|---|---|---|---|---|---|---|---|
| G. minimum group | AUS: 27 | 0.707 | p < 0.001 | DF1 to 3: 0.053 | DF1 to 3: p < 0.001 | AUS: 92.6 | AUS: 0.883* | BASDIA ORN |
| CAL: 5 | DF2 to 3: 0.235 | DF2 to 3: p < 0.001 | CAL: 100 | CAL: 1.000ns | ORMAX CODCR | |||
| GRA: 89 | DF3: 0.625 | DF3: p < 0.001 | GRA: 94.4 | GRA: 0.911* | COMCR EXEXT | |||
| MAR: 70 | MAR: 95.7 | MAR: 0.932* | ||||||
| G. glaucescens group | GLA: 28 | 0.806 | p < 0.001 | DF1 to 2: 0.082 | DF1 to 2: p < 0.001 | GLA: 78.6 | GLA: 0.633* | BASDIA ORN |
| PAP: 16 | DF2: 0.632 | DF2: p < 0.001 | PAP: 100 | PAP: 1.000* | ORNMAX CAP | |||
| PAR: 63 | PAR: 79.4 | PAR: 0.647* | EXEXT PER | |||||
| G. pectinatum group | MER: 40 | 0.794 | p < 0.001 | DF1 to 3: 0.007 | DF1 to 3: p < 0.001 | MER: 95 | MER: 0.923* | BASDIA CAP |
| PEC: 21 | DF2 to 2: 0.072 | DF2 to 3: p < 0.001 | PEC: 100 | PEC: 1.000* | CODCR STW | |||
| PLI: 11 | DF3: 0.295 | DF3: p < 0.001 | PLI: 100 | PLI: 1.000* | STL/W PER | |||
| TEN: 13 | TEN: 100 | TEN: 1.000* |
Fig. 5.
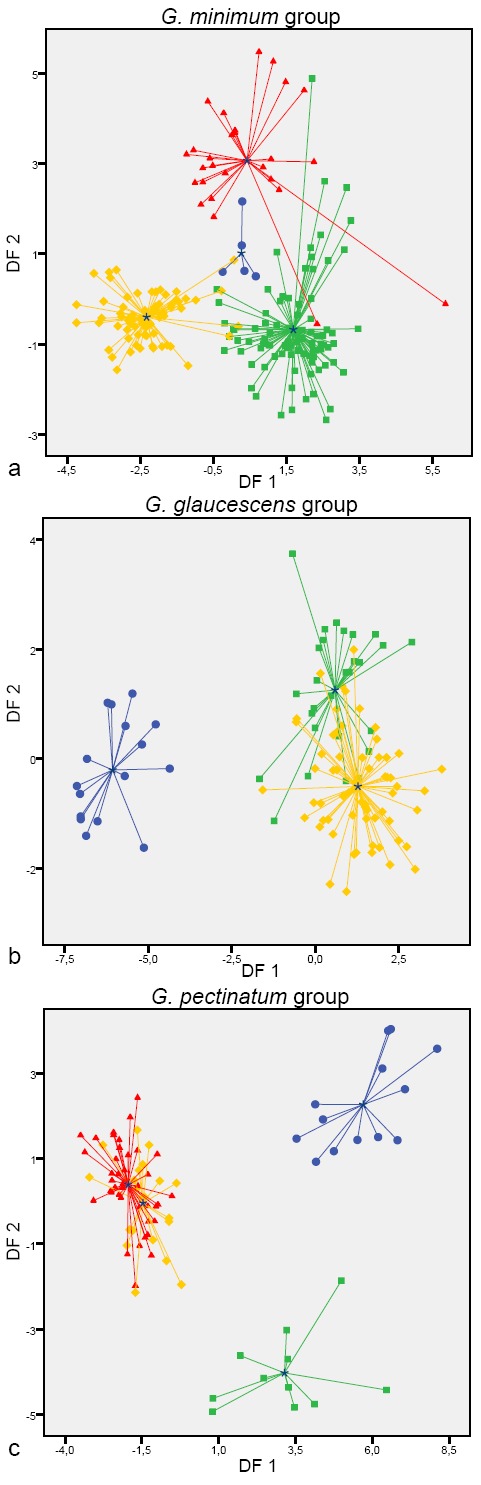
Score plots of the G. minimum, G. glaucescens, and G. pectinatum groups, using the two first discriminant functions from the discriminant function analyses. — a. G. minimum group:  G. calceum s.l.;
G. calceum s.l.;  G. granulosum;
G. granulosum;  G. marginatum;
G. marginatum;  G. austrominimum. – b. G. glaucescens group:
G. austrominimum. – b. G. glaucescens group:  G. papinuttii;
G. papinuttii;  G. glaucescens;
G. glaucescens;  G. parvistriatum. – c. G. pectinatum group:
G. parvistriatum. – c. G. pectinatum group:  G. tenuipes;
G. tenuipes;  G. plicatum;
G. plicatum;  G. pectinatum;
G. pectinatum;  G. meridionale.
G. meridionale.
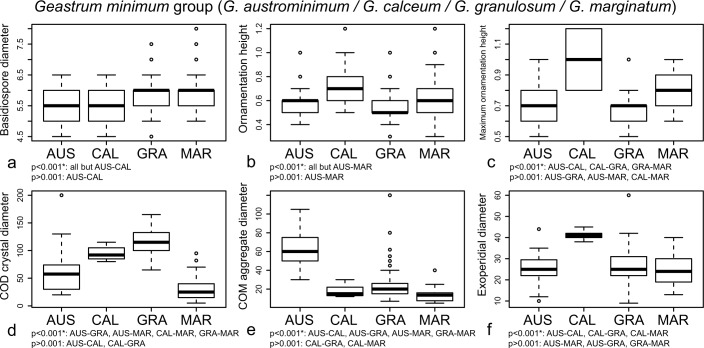
The following characters were selected as the most discriminant in the G. minimum group: basidiospore diameter, ornamentation height, maximum ornamentation height, maximum diameter of mesoperidial COD crystals, maximum diameter of mesoperidial COM aggregates, and exoperidial diameter (Table 2). All of them showed significant differences among the included species (Fig. 6). Geastrum austrominimum had the biggest mesoperidial aggregates of COM (Fig. 6e). Geastrum calceum showed the basidiomata with the largest size, represented as the exoperidial diameter (Fig. 6f), and basidiospores with the highest ornamentation, followed by G. marginatum (Fig. 6b, c). The mesoperidial COD crystals of G. granulosum and G. calceum s.l. were the biggest, and those of G. marginatum were the smallest (Fig. 6d). Significant differences were found in the basidiospore diameter, but measurements of this character also showed a broad overlapping (Fig. 6a).
Fig. 6.
Boxplots representation of the most discriminant characters retrieved by DFA for the G. minimum group, and significance indices of Tukey’s HSD tests. a. Basidiospore diameter; b. ornamentation height; c. maximum ornamentation height; d. maximum diameter of COD mesoperidial crystals; e. maximum diameter of COM mesoperidial crystal aggregates; f. exoperidial diameter. Species names abbreviated as: AUS = G. austrominimum, CAL = G. calceum s.l., GRA = G. granulosum, MAR = G. marginatum.
In the G. glaucescens group, DFA identified the following characters as the most discriminant: basidiospore diameter, ornamentation height, maximum ornamentation height, capillitium diameter, exoperidial diameter, and number of peristome folds (Table 2). Geastrum papinuttii had the smallest and less ornamented basidiospores (Fig. 7a, b, c), as well as the thinnest capillitial hyphae (Fig. 7d) and the smallest basidiomata, represented by the exoperidial diameter (Fig. 7e). The number of peristome folds did not show significant differences in any comparison pairs (Fig. 7f). Significant differences between G. glaucescens and G. parvistriatum were only found in basidiospore diameter, although boxplots showed a complete overlap of measurements (Fig. 7a).
Fig. 7.
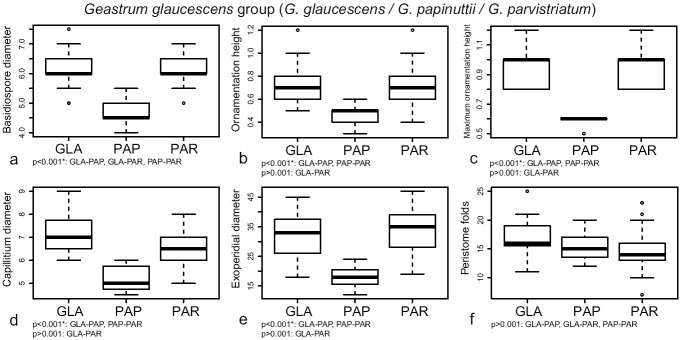
Boxplots representation of the most discriminant characters retrieved by DFA for the G. glaucescens group, and significance indices of Tukey’s HSD tests. a. Basidiospore diameter; b. ornamentation height; c. maximum ornamentation height; d. maximum diameter of the capillitial hyphae; e. exoperidial diameter; f. number of peristome folds. Species names abbreviated as: GLA = G. glaucescens, PAP = G. papinuttii, PAR = G. parvistriatum.
For the G. pectinatum group, the most discriminant morphological characters were the basidiospore size, capillitium diameter, maximum diameter of mesoperidial COD crystals, stalk width, stalk length/stalk width ratio, and number of peristome folds (Table 2). Basidiospores of G. plicatum were significantly smaller than in the remaining species (Fig. 8a). The capillitium of G. pectinatum was significantly wider than in the other species (Fig. 8b). Geastrum tenuipes showed mesoperidial crystals of COD significantly larger than other species (Fig. 8c). Geastrum meridionale had the thickest and stoutest stalks, followed by G. pectinatum, while stalks of G. plicatum and G. tenuipes were significantly thinner and more slender (Fig. 8d, e). Finally, the number of peristome folds was significantly higher in G. meridionale than in the other species (Fig. 8f).
Fig. 8.
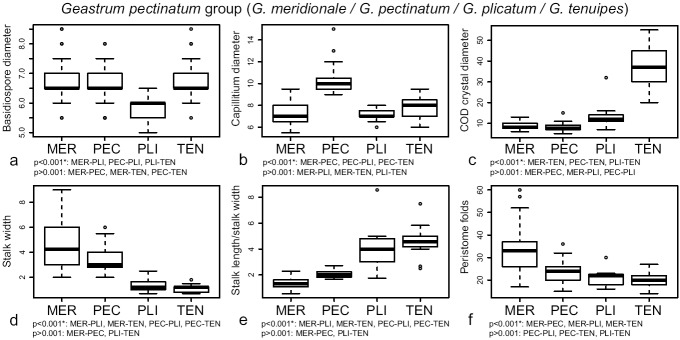
Boxplots representation of the most discriminant characters retrieved by DFA for the G. pectinatum group, and significance indices of Tukey’s HSD tests. a. Basidiospore diameter; b. maximum diameter of the capillitial hyphae; c. maximum diameter of COD mesoperidial crystals; d. stalk width; e. stalk length/stalk width ratio; f. number of peristome folds. Species names abbreviated as: MER = G. meridionale, PEC = G. pectinatum, PLI = G. plicatum, TEN = G. tenuipes.
TAXONOMY
Geastrum section Geastrum
Type. Geastrum coronatum Pers. (proposed as conserved type for Geastrum by Zamora (2014)).
Key to subsections in Geastrum section Geastrum
1. Peristome always sulcate; mostly conical to narrowly conical; mesoperidium as a very thick, often farinose, layer of pruina made up by generative hyphae and rather small crystals (mostly < 20 μm diam, except G. tenuipes up to 55 μm diam) . . . . . . . . . . . . . . . G. subsect. Sulcostomata
1. Peristome often fibrillose, rarely sulcate; mostly flat to broadly conical; mesoperidium different . . . . . . . . . . . . . . . . . . . .2
2. Basidiospores mostly 3.0–5.0 μm diam and inconspicuously ornamented (ornamentation mostly 0.1–0.5 μm high) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. subsect. Arenaria
2. Basidiospores mostly 4.5–7.0 μm diam and conspicuously ornamented (ornamentation mostly 0.4–1.0 μm high) . . . 3
3. Exoperidium ± saccate, strongly hygrometric; pseudoparenchymatous layer very persistent, made up by thick-walled (> 1.5 μm thick) hyphal cells; endoperidial body sessile to nearly so . . . . . . . . . . . . . . . . . . . . . G. subsect. Hungarica
3. Exoperidium not saccate, not hygrometric; pseudoparenchymatous layer evanescent, made up by thin-walled (mostly ≤ 1.0 μm thick) hyphal cells; endoperidial body distinctly stalked (stalk sometimes short) . . . . . . . . . . . . . . . . . . . .4
4. Basidiospores with processes not covered with warts or only with some irregular and inconstant warts; basidiomata often delicate and slender, rarely stout; mesoperidium with crystalline matter predominant over the generative hyphae (sometimes crystals and hyphae both abundant), often with crystalline aggregates of COM; peristome always fibrillose . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. subsect. Quadrifida
4. Basidiospores with processes densely covered by regular and small rounded warts; basidiomata rather stout; mesoperidium with generative hyphae predominant over the crystals (sometimes only generative hyphae present), without crystalline aggregates of COM; peristome fibrillose to sulcate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. subsect. Geastrum
Geastrum subsect. Arenaria J.C. Zamora in Zamora et al., Taxon 63, 3: 490. 2014
Key to species in Geastrum subsection Arenaria
1. Stalk stout, ≤ 1.0 mm high . . . . . . . . . . . . . . . . . . . . . . . .2
1. Stalk more or less slender, ≥ 1.3 mm high . . . . . . . . . . . . 4
2. Exoperidium fornicate; largest mesoperidial crystals 40–60 μm diam; species growing among mosses on bark of living trees . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. leptospermum
2. Exoperidium saccate to arched; largest mesoperidial crystals 10–35 μm diam; species growing in rather dry habitats and sandy soils . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Pseudoparenchymatous layer persistent, made up by more or less thick-walled (mostly 1.0–2.0 μm thick) hyphal cells; exoperidium hygrometric; American species G. arenarium
3. Pseudoparenchymatous layer rather evanescent, made up by thin-walled (mostly ≤ 1.0 μm thick) hyphal cells; exoperidium not to falsely hygrometric; South European species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. benitoi
4. Basidiospores mostly globose to subglobose, (3.5–)4.0– 4.5(–5.0) μm diam; ornamentation 0.3–0.5(–0.6) μm high; exoperidium fornicate; Paleartic species . . G. britannicum
4. Basidiospores mostly ovoid, 3.0–4.0 × 2.5–3.5 μm; ornamentation 0.1–0.3 μm high; exoperidium arched; Neotropical species . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. ovalisporum
Geastrum arenarium Lloyd, Bull. Lloyd Libr. Bot. 5: 28. 1902, ‘Geaster arenarius’ — Fig. 3, 9a
Type. Lectotype (designated here as a ‘second-step’ lectotype, MBT 198515): USA, Florida, Jupiter, Feb. 1895, H.C. Culbertson, Smithsonian Institution 57292, BPI 704841!, excluding one basidioma of G. quadrifidum and one basidioma of Astraeus sp. (as A. hygrometricus) that are marked separately.
Synoptic description (based on Sunhede (1986) and studied specimens) — Exoperidium 15–40 mm diam in horizontal position, saccate to arched when hydrated, with (6–)7–10(–13) hygrometric rays. Endoperidial body 5–19 mm diam, whitish to grey or brownish grey. Mesoperidium as a thin layer of pruina, sometimes inconspicuous. Largest mesoperidial crystals of COD on the endoperidial surface 15–30 μm diam, bipyramidal. Peristome fibrillose, distinctly delimited. Stalk mostly 0.2–1.0 mm high, rarely indistinct, whitish. Basidiospores mostly globose to subglobose, some ovoid, 3.5–4.5(–5.0) μm diam, with (0.2–)0.3–0.4(–0.7) μm high warts, ornamentation verrucose. Pseudoparenchymatous layer persistent, of more or less thickwalled (mostly 1–2 μm thick) cells.
Ecology & Distribution — Originally described from sandy soils of the ‘Temperate conifer forests’ biome of the Neartic ecozone (Lloyd 1902). It has been also found in ‘Deserts and xeric shrublands’ and ‘Tropical and subtropical grasslands, savannas and shrublands’ biomes of the Neartic (Bates 2004) and Neotropical (Kuhar et al. 2013) ecozones. Unconfirmed records have been also made from the Afrotropical (Bottomley 1948) and Australasian (Cunningham 1944) ecozones.
Additional specimens examined. ARGENTINA, Catamarca, camino a ‘Chañaritos’, entre mantillo, bajo jarilla y quebracho blanco, Chaco serrano árido, 2 Mar. 1994, M.M. Dios, G. Castro & L. Rodríguez, BAFC 33321; Córdoba, Pocho, reserva Chancaní, cerca de la vivienda, bajo Prosopis chilensis, 7 June 1993, L. Domínguez & E. Crespo 1333, CORD 1333; La Rioja, Miranda, Prosopis sp., 27 Mar. 2008, L. Papinutti & G. Rolón, MA-Fungi 83760. – USA, Arizona, 7 mi. from Nogales, 11 Sept. 1941, Long & Stouffer 9634, LPS 29761; Arizona, 10 mi. from Nogales, along Tucson-Nogales highway, 4 June 1930, Long 8304, LPS 29751; Arizona, Tucson, near Sabino Canyon, 4 June 1938, W.H. Long & Stouffer 9263, LPS 29763; Florida, locis arenosis, C.G. Lloyd 213, S.
Nomenclatural notes — Sunhede (1986) firstly lectotypified the species with BPI 704841, but without explicitly selecting a part of this collection or explicitly excluding the non-conform species (Art. 9.17).
Geastrum benitoi J.C. Zamora, sp. nov. — MycoBank MB810499; Fig. 3, 9b
Type. SPAIN, Madrid, Villaviciosa de Odón, urbanización Campodón, under Olea europaea, on sandy, siliceous soil, 12 Nov. 2011, B. Zamora & J.C. Zamora, Zamora 499, holotype MA-Fungi 87324!, isotypes in AH 45201! And UPS!
Etymology. The specific epithet is dedicated to Benito Zamora, father of the first author, who has been helping him to collect Geastrum specimens during years of field trips.
Diagnosis — Exoperidium (13–)15–35(–38) mm diam in horizontal position, arched, with (6–)7–11(–13) often falsely hygrometric rays. Endoperidial body (4–)5–11(–13) mm diam, mostly greyish cream to brownish grey, rarely whitish. Mesoperidium as a thin layer of pruina. Largest mesoperidial crystals of COD on the endoperidial surface 18–35 μm diam, often bipyramidal prisms with well-developed faces, but also bipyramids. Peristome fibrillose, well-delimited. Stalk 0.2–1.0 mm high, whitish to cream. Basidiospores mostly globose to subglobose, (3.5–)4.0–5.0(–5.5) μm diam, with 0.3–0.5(–0.6) μm high warts, ornamentation verrucose. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells. Rhizomorphs with bipyramidal crystals of COD, isolated or grouped in rose-like aggregates.
Macroscopic characteristics — Unexpanded basidiomata 4–7 mm diam, subglobose, with a rounded apex or a flat umbo. Exoperidium splitting in (6–)7–11(–13) more or less equal to unequal rays, (9–)10–30(–33) mm diam apparently, (13–)15– 35(–38) mm diam when forced in horizontal position, arched, often falsely hygrometric. Mycelial layer thin, whitish to pale cream, strongly intermixed with debris from the substrate, more or less strongly adhered to the fibrous layer, but sometimes peeling-off in some parts. Fibrous layer papyraceous to slightly coriaceous when denuded, whitish to pale-cream coloured. Pseudoparenchymatous layer pale cream to greyish cream, not or only superficially cracked, < 0.5 mm thick when dried, about 1 mm thick when fresh, more or less evanescent. Endoperidial body globose to subglobose, rarely irregular, (4–)5–11(–13) mm diam, greyish cream to whitish; endoperidial surface glabrous or almost so, in newly expanded basidiomata covered with very small, bipyramidal crystals, often in the form of bipyramidal prisms with conspicuous faces, bipyramids also present, gradually disappearing. Peristome fibrillose, mostly darker than the endoperidial surface, broadly conical to almost flat, 0.5–1.5 mm high, well-delimited. Stalk present but often very short, 0.2–1.0 mm high, whitish. Apophysis absent or poorly developed, concolorous with the endoperidium. Columella intruding about 1/3–1/2 into the glebal mass. Mature gleba dark greyish brown.
Microscopic characteristics — Basidia narrowly ellipsoid to subcylindric or more or less lageniform, 15–23 × 4.5–6.5 μm, with (3–)4–7(–8) short sterigmata. Basidiospores mostly globose to subglobose, a few ovoid, (3.5–)4.0–5.0(–5.5) μm diam, brownish to yellowish brown, with 0.3–0.5(–0.6) μm high brown, irregular warts, ornamentation verrucose. Broadest capillitial hyphae 4.5–6.5 μm wide, aseptate, very rarely branched, normally straight, thick-walled (2.0–3.0 μm thick), with narrow lumen, mostly visible; tips acute to rounded; surface covered with debris or not. Endoperidium composed of 2.0–5.0(–6.0) μm wide, yellowish to yellowish brown, aseptate, mostly unbranched, slightly sinuous, strongly intertwined, thick-walled hyphae, lumen visible; protruding hyphae absent or very sparse and almost indistinct. Peristomal hyphae 2.5–7.5 μm wide, light brown, aseptate, mostly unbranched, thick-walled (1.0–2.5 μm thick), lumen visible, straight to somewhat sinuous, narrowing at base and apex, tips mostly acute to more or less rounded. Mesoperidium present on the endoperidium and pseudoparenchymatous layer surfaces, consisting of 18–35 μm diam, bipyramidal crystals of COD, intermixed with some 1.0–3.0 μm wide, hyaline, branched, thin-walled, clamped hyphae. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick), hyaline to yellowish cells, variable in shape and size, about 12–75 × 10–32 μm. Fibrous layer with 1.5–5 μm wide, hyaline to very pale yellowish, aseptate, straight or slightly sinuous, intertwined, mostly unbranched, comparatively thick-walled (0.5–2.0 μm thick) hyphae, lumen more or less visible. Mycelial layer double-layered; inner layer consisting of 1.5–3 μm wide, strongly glued together, more or less hyaline, branched, thin-walled and clamped hyphae; outer layer with 1–3.5 μm wide, hyaline to somewhat yellowish, aseptate, rarely branched, comparatively more or less thick-walled (0.5–1.5 μm thick) hyphae, lumen hardly visible. Rhizomorphs covered with rose-like aggregates of bipyramidal crystals.
Ecology & Distribution — It is only known from sandy, siliceous soils of the Iberian Peninsula, which is part of the ‘Mediterranean forests, woodlands and scrub’ biome of the Paleartic ecozone.
Additional specimens examined (paratypes). SPAIN, Badajoz, Campanario, Badija, open area, sandy, siliceous soil, 2 Nov. 2013, M. Gordillo, herb. Zamora 615; Jaén, Santuario de la Virgen de la Cabeza, en jaral, 30 Dec. 1984, A.G. Buendía, MA-Fungi 8106; Madrid, Casa de Campo, 6 Dec. 1985, M. Jeppson, MA-Fungi 16940; Madrid, Colmenarejo, río Aulencia, 30TVK1582, 635 m, en pradera, 20 Oct. 2001, F. Prieto GP292, MA-Fungi 68191; ibid., suelo arenoso, casi desnudo, en las proximidades de Retama sphaerocarpa y Quercus rotundifolia, 28 Jan. 2002, F. Prieto, herb. Zamora 208; ibid., 20 Feb. 2002, F. Prieto & Á. González, herb. Zamora 209; ibid., 4 Jan. 2007, F. Prieto & J.C. Zamora, herb. Zamora 210; Madrid, Villaviciosa de Odón, urbanización Campodón, bajo olivos en un antiguo olivar abandonado, 17 Mar. 2001, J.C. Zamora, MA-Fungi 53523 (duplo herb. Zamora 13); ibid., under Olea europaea, on sandy, siliceous soil, 27 Feb. 2005, J.C. Zamora, herb. Zamora 76; ibid., 14 Dec. 2005, B. Zamora & J.C. Zamora, herb. Zamora 124, ibid., 20 Oct. 2006, J.C. Zamora, herb. Zamora 180; ibid., 3 Feb. 2007, B. Zamora & J.C. Zamora, herb. Zamora 214; ibid., 8 Dec. 2008, B. Zamora, J. Señoret & J.C. Zamora, herb. Zamora 317; ibid., 24 Dec. 2010, B. Zamora, J.C. Zamora & J.C. Campos, herb. Zamora 470; ibid., 24 Dec. 2010, J.C. Zamora, B. Zamora & J.C. Campos, herb. Zamora 471; ibid., 6 Nov. 2011, B. Zamora, herb. Zamora 498; ibid., 28 Dec. 2011, B. Zamora & J.C. Zamora, herb. Zamora 500; ibid., 21 Oct. 2012, B. Zamora, J. Señoret & J.C. Zamora, herb. Zamora 542; ibid., 15 Mar. 2014, B. Zamora & J.C. Zamora, herb. Zamora 615.
Geastrum britannicum J.C. Zamora, sp. nov. — MycoBank MB810500; Fig. 3, 9c
Type. UK (ENGLAND), Norfolk, Cockley Cley, in litter, Pinus sylvestris, 25 Sept. 2000, J. Revett, holotype K(M) 79617!
Etymology. The specific epithet refers to the only country where the species is known from.
Diagnosis — Exoperidium 44–52 mm diam in horizontal position, fornicate, with 4–7 non-hygrometric rays. Endoperidial body 11–12 mm diam, pale to dark brown. Mesoperidium wellformed, with abundant crystalline matter. Largest mesoperidial crystals of COD on the endoperidial surface 45–80 μm diam, bipyramidal, often grouped in 60–120 μm diam rounded aggregates. Peristome fibrillose, well-delimited. Stalk 2.0–3.0 mm high, brownish. Basidiospores globose to slightly ovoid, (3.5–)4–4.5(–5) μm diam, with (0.3–)0.4–0.5(–0.6) μm high warts, ornamentation verrucose. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Macroscopic characteristics — Unexpanded basidiomata not seen. Exoperidium split in 4–7 more or less equal to unequal rays, 30–50 mm diam apparently, 44–52 mm diam when forced in horizontal position, fornicate when mature, not hygrometric. Mycelial layer comparatively thick, whitish to pale cream, strongly intermixed with debris from the substrate, separating from the fibrous layer and remaining as an irregular mycelial cup attached to the tips of the rays. Fibrous layer more or less coriaceous when denuded, whitish to cream coloured. Pseudoparenchymatous layer pale cream to ochraceous cream, frequently cracked, less than 1 mm thick in dry state, peeling off from the fibrous layer and not very persistent (absent in old basidiomata). Endoperidial body subglobose to ovoid, sometimes irregular, 11–12 mm diam, pale to dark brown; endoperidial surface glabrous or almost so, in newly expanded basidiomata densely covered with big, bipyramidal crystals, often forming rounded aggregates, remaining long time and gradually disappearing. Peristome fibrillose but with up to 10 inconspicuous and poorly developed folds near the centre, mostly darker than the endoperidial surface, broadly conical to almost flat, 1.0–1.5 mm high, sharply delimited. Stalk present, well-developed, 2.0–3.0 mm high, brownish. Apophysis well-defined, concolorous or slightly lighter than the endoperidium. Columella intruding about 1/2 into the glebal mass. Mature gleba dark greyish brown.
Microscopic characteristics — Basidia not seen. Basidiospores globose to slightly ovoid, (3.5–)4.0–4.5(–5.0) μm diam, brownish to yellowish brown, with (0.3–)0.4–0.5(–0.6) μm high brown warts, ornamentation verrucose, hilar appendix 0.5–0.8 μm long. Broadest capillitial hyphae 3.5–9.5 μm wide, aseptate, very rarely branched, normally straight, thick-walled (1.5–4.5 μm thick), with narrow lumen, mostly visible; tips acute to rounded; surface often covered with debris. Endoperidium composed of 1.5–5.0 μm wide, pale yellowish to yellowish brown, aseptate, mostly unbranched, slightly sinuous, strongly intertwined, thick-walled hyphae, lumen visible; protruding hyphae not seen. Peristomal hyphae 3.0–5.5 μm wide, brown to dark brown, aseptate, mostly unbranched, thick-walled (1.0–2.5 μm thick), lumen mostly visible, often sinuous, narrowing at base and apex, tips mostly rounded, a few acute; abundant debris present among hyphae. Mesoperidium present on the endoperidium and pseudoparenchymatous layer surfaces, consisting of rather large, bipyramidal crystals, 20–80 μm diam (the largest 63–80 μm diam), usually grouped to form 60–120 μm diam rounded aggregates, intermixed with some 1.5–3.0 μm wide, hyaline, branched, thin-walled, clamped hyphae. Pseudoparenchymatous layer of thin-walled (≤ 1 μm thick), hyaline to yellowish cells, variable in shape and size, about 20–60 × 15–40 μm. Fibrous layer with 2.0–4.5 μm wide, hyaline to very pale yellowish, aseptate, straight or slightly sinuous, intertwined, mostly unbranched, comparatively thick-walled (1.0–1.5 μm thick) hyphae, lumen visible. Mycelial layer double-layered; inner layer consisting of 2.0–4.0 μm wide, strongly glued together, more or less hyaline, branched, thin-walled and clamped hyphae; outer layer with 1.5–2.5 μm wide, hyaline to somewhat yellowish, aseptate, rarely branched, comparatively more or less thick-walled (0.5–1.5 μm thick) hyphae, lumen very narrow and difficult to perceive; myceliar projections indistinct from the outer myceliar layer. Rhizomorphs not studied.
Ecology & Distribution — Known from only three specimens collected in humid soils of the ‘Temperate broadleaf and mixed forests’ of the Paleartic ecozone.
Additional specimens examined (paratypes). UK (ENGLAND), Hampshire, nr. New Milton, soil, Taxus, 2 Feb. 1995, D. & M. Nesbitt, K(M) 60288; Norfolk, Surlingham, on soil, Quercus sp., June 2002, T.W. Dove, K(M) 99914.
Geastrum leptospermum G.F. Atk. & Coker in Atkinson, Bot. Gaz. 36: 306. 1903, ‘Geaster leptospermus’ — Fig. 3, 9d
Type. Lectotype (designated here, MBT198516): USA, North Carolina, Chapel Hill, on mosses covered living trunks of trees, Feb. 1903, W.C. Coker 2644, Atkinson Herbarium of Cornell University 14861b (slide) and 14861c (basidiomata), CUP! (since the collector number is the same in both herbarium envelopes, we consider them as a single gathering).
Synoptic description (based on Atkinson (1903), Coker & Couch (1928), Sunhede (1989), and the type specimens studied) — Exoperidium 8–12 mm diam in horizontal position, fornicate, with (3–)4–7 non-hygrometric rays. Endoperidial body 2.5–5(–6.5) mm diam, pale to dark brown. Mesoperidium thin, with numerous but more or less sparse crystals. Largest mesoperidial crystals of COD on the endoperidial surface 40–60 μm diam, bipyramidal. Peristome fibrillose, well-delimited. Stalk 0.2–0.5 mm high, whitish. Basidiospores globose to slightly ovoid, 3.0–4.0 μm diam, with (0.1–)0.2–0.3 μm high warts, ornamentation verrucose. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells. Rhizomorph crystals mainly oblique prisms of COM, grouped in stellate aggregates; bipyramidal crystals of COD also observed.
Ecology & Distribution — This rare species grows on mossy bark of living trees, and is only known from North Carolina (Atkinson 1903, Coker & Couch 1928), being found in the ‘Temperate broadleaf and mixed forests’ biome of the Neartic ecozone.
Additional specimens examined. USA, North Carolina, Chapel Hill, on mosses covered living trunks of tree, woods, Feb. 1902 [probably an error for 1903], W.C. Coker 2319, Atkinson Herbarium of Cornell University 14861a, CUP (syntype).
Geastrum ovalisporum Calonge & Mor.-Arr. in Calonge et al., Bol. Soc. Micol. Madrid 25: 273. 2000 — Fig. 9e
Type. BOLIVIA, Concepción, Piedra de Santa Teresita, en claros de bosque tropical, sobre suelo arenoso con abundantes restos vegetales en descomposición, 5 Mar. 2000, B. Moreno-Arroyo & J. Gómez, holotype MA-Fungi 47184!
Synoptic description (based on Calonge et al. (2000) and studied specimens) — Exoperidium 26–45 mm diam in horizontal position, arched, with 5–8 non-hygrometric rays. Endoperidial body 8–15 mm diam, brown to almost black. Mesoperidium thin, with rather sparse crystals. Largest mesoperidial crystals of COD on the endoperidial surface 37.5–78 μm diam, bipyramidal (bipyramids and bipyramidal prisms), sometimes irregular. Peristome fibrillose, well-delimited. Stalk 1.3–2.0 mm high, whitish. Basidiospores ovoid, 3.0–4.0 × 2.5–3.5 μm, with 0.1–0.3 μm high warts, ornamentation verrucose, inconspicuous. inconspicuous. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells. Rhizomorphs with bipyramidal crystals of COD, isolated or grouped in rose-like aggregates, and also some horn-like crystals of COM.
Ecology & Distribution — Only known from South America (Calonge et al. 2000, Cortez et al. 2008). It grows on humic soils of the ‘Tropical and subtropical moist broadleaf forests’ biome in the Neotropic ecozone.
Additional specimens examined. ARGENTINA, Salta, La Candelaria, Palo Quemado, Yungas, 12 Apr. 2012, L. Papinutti, G. Rolón & J.C. Zamora, MA-Fungi 86670. – BRAZIL, Rio Grande do Norte, Parque Estadual Dunas do Natal, sobre solo arenoso, 10 July 2004, I.G. Baseia, URFN-Fungos 229.
Geastrum subsect. Geastrum
Key to species in Geastrum subsect. Geastrum
1. Peristome clearly sulcate, with thick folds . . . . . . . . . . . .2
1. Peristome fibrillose or transition between fibrillose and very finely sulcate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2. Basidiospores (4.8–)5.0–6.0(–6.5) μm, stalk mostly broader than long, American species . . . . . . . . . . . . . . . G. smithii
2. Basidiospores 5.5–7.0 μm, stalk slightly longer than broad, Australasian species . . . . . . . . . . . . . . . . . . . G. aff. Smithii
3. Rhizomorph crystals mostly horn-like; mesoperidium as a mealy cover made up mainly by generative hyphae, with scarce and small bipyramidal crystals (sometimes up to 75 μm diam, well-formed crystals, appear); stalk (1.0–)2.0– 5.0 mm high; peristome always fibrillose, indistinctly to more or less distinctly delimited; broadest capillitial hyphae (5.0–) 5.5–11.0(–15.0) μm wide . . . . . . . . . . . . . . . G. coronatum
3. Rhizomorph crystals mostly bipyramidal, grouped in roselike aggregates or not; mesoperidium formed by generative hyphae and numerous bipyramidal crystals up to 127.5 μm diam; stalk 1.0–2.5 mm high; peristome fibrillose to finely sulcate, mostly distinctly delimited; broadest capillitial hyphae (4.0–)4.5–5.5 μm wide . . . . . . . . . G. thanatophilum
Geastrum coronatum Pers., Syn. Meth. Fung.: 132. 1801 — Fig. 4, 10a
Fig. 10.
Morphological characters in Geastrum subsect. Geastrum and Geastrum subsect. Quadrifida pro parte (G. kuharii). a. G. coronatum (Zamora 266); b. G. thanatophilum (holotype); c. G. smithii (MA-Fungi 83783); d. G. aff. smithii (CANB 748746); e. G. kuharii (holotype). — a1, b1, c1, d1, e1. Basidiomata habit; a2, b2, c2, d2, e2. detail of the peristome; a3, b3, c3, d3, e3. detail of the stalk and apophysis; a4, b4, c4, d4, e4. mesoperidial COD crystals on the endoperidial surface, in a4 almost no crystalline matter present; a5, b5, c5, d5, e5. basidiospores. — Scale bars: a1, b1, c1, d1, e1 = 5 mm; a2, b2, c2, d2, e2, a3, b3, c3, d3, e3 = 2 mm; a4, b4, c4, d4, e4 = 10 μm; a5, b5, c5, d5, e5 = 2 μm.
Type. Lectotype (designated by Demoulin 1984): f. 2 of pl. XLVI in Schmidel (1793).
≡ Geastrum multifidum Pers., Neues Mag. Bot. 1: 86. 1794.
≡ Geastrum limbatum Fr., Syst. Mycol. 3: 15. 1829, ‘Geaster limbatus’, nom. illeg., Art. 52.1.
= Geastrum atratum F. Šmarda, Česká Mykol. 1: 74. 1947, ‘Geaster atratus’. — Type: Unknown. In absence of original specimens, f. 2 in Šmarda (1947), f. 25 of t. IX in Hollós (1904), or f. 44 in Lloyd (1902) can be selected as lectotype.
= Geastrum coronatum var. moelleri V.J. Staněk in Pilát, Flora ČSR B-1: 428, 789. 1958, ‘Mülleri’ (the epithet ‘Mülleri’ was corrected according to Art. 60.6 and Rec. 60F). — Type: HUNGARY, Nagyerdö Csokás, Nagykörös, 14 Nov. 1955, I. Müller, holotype PRM 842868!
Synoptic description (based on Sunhede (1989) and studied specimens) — Exoperidium (33–)35–120(–135) mm diam in horizontal position, arched, with (5–)7–12(–15) sometimes falsely hygrometric rays. Endoperidial body 9–43 mm diam, brownish to blackish. Mesoperidium as a dense mealy cover, mostly with scarce or indistinct crystalline matter, but sometimes with welldeveloped crystals. Largest mesoperidial crystals of COD on the endoperidial surface 2–53(–75) μm diam, bipyramidal. Peristome fibrillose, distinctly or indistinctly delimited. Stalk stout, (1.0–)2.0–4.5(–5.5) mm high, brownish or greyish to almost black, rarely cream coloured. Basidiospores globose, (5.0–)5.5– 6.5(–7.0) μm diam, with 0.5–1.0 μm high warts, ornamentation verrucose. Broadest capillitial hyphae (5.0–)5.5–11.0(–15.0) μm wide. Pseudoparenchymatous layer of thinwalled (mostly ≤ 1 μm thick) cells. Rhizomorphs mainly with thin horn-like crystals of COM, forming arachnoid structures.
Ecology & Distribution — Confirmed records are known from calcareous and siliceous soils, mostly on humic places, and from several biomes (‘Boreal forests/taiga’, ‘Temperate conifer forests’, ‘Temperate broadleaf and mixed forests’, and ‘Mediterranean forests, woodlands and scrub‘) of the Neartic and Paleartic ecozones (Sunhede 1989, Calonge 1998, Bates 2004). Also reported from Paleotropical (Bottomley 1948) and Australasian (Cunningham 1944) ecozones.
Additional specimens examined. CZECH REPUBLIC, Ýmel, 1972, E. Futó, MICH 72513. – SPAIN, Madrid, Casa de Campo, enfrente del Club de Campo, bajo Pinus sp., 12 Dec. 2010, L. Penelas, herb. Zamora 464; Madrid, Villaviciosa de Odón, parque cercano al Castillo, sandy, siliceous, and humic soil, under Ulmus minor and Cupressus arizonica, 24 Dec. 2003, J.C. Zamora, herb. Zamora 37; ibid., under Ulmus minor, 19 Sept. 2006, B. Zamora & J.C. Zamora, herb. Zamora 158; ibid., under Quercus robur, 28 Oct. 2006, J.C. Zamora, herb. Zamora 181; ibid., under Cupressus sempervirens, Ulmus minor, Populus sp., Calocedrus sp., and Celtis australis, siliceous soil, 25 Nov. 2007, B. Zamora & J.C. Zamora, herb. Zamora 266; ibid., under Cupressus sp., Cedrus sp., Ulmus minor, and Populus sp., 27 Dec. 2009, S. Pardillo, P.L. Aznar & J.C. Zamora, herb. Zamora 364; ibid., under Quercus robur, 24 Dec. 2010, B. Zamora, J.C. Zamora & J.C. Campos, herb. Zamora 476; ibid., under Ulmus minor and Pinus sp., 7 May 2011, B. Zamora, J. Señoret & J.C. Zamora, herb. Zamora 484; ibid., under Ulmus minor and Cupressus sempervirens, 13 Nov. 2011, B. Zamora & J.C. Zamora, herb. Zamora 549; ibid., under Cupressus sp. and Ulmus minor, 13 Nov. 2011, B. Zamora & J.C. Zamora, herb. Zamora 576. – SWEDEN, Gotland, Bunge parish, Bunn, dead anthill, 5 Dec. 1970, S. Sunhede, herb. Sunhede 7601; Gotland, Gothem parish, Jusarve skog, on an anthill, mixed forest with Pinus sylvestris, Picea abies, and Quercus robur, on calcareous soil, 30 Sept. 2011, J.C. Zamora, herb. Zamora 522. – USA, Arizona, Coconino Co., Walnut Canyon National Monument, Pinus edulis and Juniperus, 3 May 1995, J. States AEF 1443, MICH 28567.
Nomenclatural notes — Demoulin (1984) made homotypic the names G. coronatum and G. multifidum by selecting one of the figures cited under ‘G. multifidum var. β’ in Persoon (1794) as type for both, while, according to Art. 32.1(b), the name ‘G. multifidum var. β’ itself cannot be considered as validly published in the infraspecific rank, see also Ex. 4 of Art. 9.5.
Geastrum smithii Lloyd, Bull. Lloyd Libr. Bot. 5: 21. 1902, ‘Geaster’ — Fig. 10c
Type. Lectotype (designated here, MBT198517): USA, Florida, New Smyrna, Mrs. Sams, Smithsonian Institution (Herb. Lloyd) 22731, BPI 705991 (photo!).
Synoptic description (based on Bates (2004) and studied specimens) — Exoperidium (14–)30–57 mm diam in horizontal position, arched, with 7–12 sometimes falsely hygrometric rays. Endoperidial body 7–23 mm diam, pale brown to blackish. Mesoperidium well-developed, with abundant big and small crystals. Largest mesoperidial crystals of COD on the endoperidial surface about (20–)50–100 μm diam, bipyramidal. Peristome sulcate, sharply delimited, normally flat to very broadly conical, with 16–30 folds. Stalk stout, 1.5–3.0 mm high, light to dark brown. Basidiospores globose, (4.8–)5.0–6.0(–6.5) μm diam, with (0.4–)0.5–0.7(–0.8) μm high warts, verrucose ornamentation. Broadest capillitial hyphae 5.0–6.5 μm diam. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Known from ‘Temperate conifer forests’, ‘Tropical and subtropical grasslands, savannas and shrublands’, and ‘Deserts and xeric shrublands’ biomes, of the Neartic and Neotropic ecozones (Lloyd 1902, Bates 2004). Records from the Australasian ecozone (Cunningham 1944) probably belong to a different taxon (see below).
Additional specimens examined. ARGENTINA, Córdoba, Cruz del Eje, Villa de Soto, Cupressus sp., 6 Apr. 2012, L. Papinutti, J.C. Zamora & G. Rolón, MA-Fungi 83783; Córdoba, Punilla, Hayke, a 3 km de Icho Cruz, por ruta provincial N°14, sobre mantillo bajo espinillo, 20 Jan. 1990, L. de Toledo 843, CORD 843; La Rioja, Sañogasta, S29°18'51.43" W67°35'37.36", on abundant leaf litter of diverse herbaceous plant species, 27 Mar. 2008, L. Papinutti & G. Rolón, BAFC 51945. – USA, New Mexico, 6 mi. W of Corona, 4 Sept. 1941, Long & Stouffer 9505, LPS 29986; ibid., 15 Sept. 1941, Long & Stouffer 9662, LPS 29801; New Mexico, 2 mi. NE of Corona, Long & Stouffer, 17 Apr. 1942, LPS 29979; ibid., 14 Jan. 1942, Long & Stouffer 9993, LPS 30189.
Geastrum aff. smithii Lloyd — Fig. 10d
Synoptic description — Exoperidium 42–47 mm diam in horizontal position, arched, with 8–9 not hygrometric or falsely hygrometric rays. Endoperidial body 13–15 mm diam, dark brownish grey. Mesoperidium well-developed, with abundant crystals. Largest mesoperidial crystals of COD on the endoperidial surface 120–160 μm diam, bipyramidal. Peristome sulcate, sharply delimited, flat to very broadly conical, with 25–29 folds. Stalk stout, 2.0–2.5 mm high, light to dark brown. Basidiospores globose, 5.5–7.0 μm diam, with 0.5–0.8 μm high warts, verrucose ornamentation. Broadest capillitial hyphae 5.0–5.5 μm diam. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Insufficiently known; a single herbarium collection from the ‘Temperate grasslands, savannas and shrublands’ biome of the Australasian ecozone has been studied.
Additional specimens examined. AUSTRALIA, New South Wales, Riverina, Ringwood State Forest, SANDS Plot 26, 11 June 2001, J. Trappe 26384A, CANB 748746 (duplo in OSC).
Geastrum thanatophilum J.C. Zamora, sp. nov. — MycoBank MB810501; Fig. 4, 10b
Type. USA, Wisconsin, Dane Co., Mazomanie cemetery, between old juniper and a headstone, 29 Sept. 2007, J.J. Steinke & P. Watson, holotype MICH 72012!
Etymology. From the Greek words Θάνατος (transliterated ‘Thanatos’, god of death) and φίλος (transliterated ‘philos’, friend), because the two studied collections came both from cemeteries.
Diagnosis — Exoperidium 22–46 mm diam in horizontal position, arched, with 6–11(–14) sometimes falsely hygrometric rays. Endoperidial body 7–16 mm diam, greyish cream to brownish grey. Mesoperidium as a dense mealy cover with big crystals. Largest mesoperidial crystals of COD on the endoperidial surface 55–127.5 μm diam, bipyramidal. Peristome fibrillose to obscurely sulcate, then with up to 35 folds, mostly well-delimited. Stalk stout, 1.0–2.5 mm high, normally greyish to rather dark brown. Basidiospores globose, (4.5–)5.0–6.0(–6.5) μm diam, with (0.3–)0.4–0.8(–1.0) μm high warts, ornamentation verrucose. Broadest capillitial hyphae (4.0–)4.5–5.5 μm wide. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1.2 μm thick) cells. Rhizomorphs with bipyramidal crystals of COD, isolated or grouped in rose-like aggregates.
Macroscopic characteristics — Unexpanded basidiomata 15–26 mm diam, subglobose, with a rounded apex or a flat umbo. Exoperidium splitting in 6–11(–14) subequal to unequal rays, 9–41 mm diam apparently, 22–46 mm diam when forced in horizontal position, arched, not truly hygrometric but young rays may bent towards the endoperidial body when the mycelial layer peels off. Mycelial layer thick, whitish to pale cream, strongly intermixed with debris from the substrate, more or less strongly adhered to the fibrous layer but peeling-off in some parts. Fibrous layer more or less papyraceous to coriaceous when denuded, whitish to pale-cream coloured. Pseudoparenchymatous layer pale cream to greyish cream, mostly uncracked, < 0.5 mm thick in dry state, about 1.5–2.5 mm thick when hydrated, not persisting in old basidiomata. Endoperidial body globose to subglobose, rarely irregular, mostly wider than high, 7–16 mm diam, greyish cream to brownish grey; endoperidial surface glabrous or almost so, in newly expanded basidiomata covered with abundant collapsed hyphae and some big, bipyramidal crystals, gradually disappearing.
Peristome fibrillose to somewhat sulcate, with 0–35 obscure folds, concolorous with the endoperidial surface, broadly conical to almost flat, up to 1 mm high, mostly distinctly delimited but specimens with indistinctly delimited peristomes occur. Stalk present, comparatively stout, 1.0–2.5 mm high, greyish to rather dark brown, very rarely pale coloured. Apophysis absent or poorly developed, concolorous with the endoperidium. Columella intruding about 1/3–1/2 into the glebal mass. Mature gleba dark greyish brown.
Microscopic characteristics — Basidia narrowly ellipsoid, more or less lageniform to more or less lecythiform, (12.5–)14– 20 × 5.0–7.5 μm, with 4–8 short sterigmata. Basidiospores globose to subglobose, (4.5–)5.0–6.0(–6.5) μm diam, brownish to yellowish brown, with (0.3–)0.4–0.8(–1.0) μm high brown warts, ornamentation verrucose. Broadest capillitial hyphae (4.0–)4.5–5.5 μm wide, aseptate, very rarely branched, normally straight, thick-walled (2–2.5 μm thick), with narrow lumen, more or less visible; tips acute to rounded; surface often covered with debris. Endoperidium composed of 2.0–7.0 μm wide, yellowish to pale brownish, aseptate, mostly unbranched, slightly sinuous, strongly intertwined, thick-walled hyphae, with lumen mostly visible; protruding hyphae not seen. Peristomal hyphae 2.5–8.0 μm wide, yellowish brown to brownish, aseptate, mostly unbranched, thick-walled (1.0–3.0 μm thick), lumen often visible, more or less straight to rather sinuous, narrowing at base and apex, tips mostly rounded, a few acute. Mesoperidium present on the endoperidium and pseudoparenchymatous layer surfaces, consisting of rather large, bipyramidal crystals of COD, the largest 55–127.5 μm diam, intermixed with abundant 1.5–4.0 μm wide, hyaline, branched, thin-walled, clamped hyphae. Pseudoparenchymatous layer of thin- to slightly thick-walled (0.5–1.2 (–1.5) μm thick), hyaline to yellowish cells, variable in shape and size, about 15–112 × 11–50 μm diam. Fibrous layer with 1.5–4.5(–5.0) μm wide, hyaline to very pale yellowish, aseptate, straight or slightly sinuous, intertwined, mostly unbranched, comparatively thick-walled (0.5–2.0 μm thick) hyphae, lumen more or less visible. Mycelial layer doublelayered; inner layer consisting of 2.0–3.0 μm wide, strongly glued together, more or less hyaline, branched, thin-walled and clamped hyphae; outer layer with 1.5–4.0 μm wide, hyaline to somewhat yellowish, aseptate, rarely branched, comparatively more or less thick-walled (0.7–2.0 μm thick) hyphae, lumen often very narrow and difficult to observe. Rhizomorphs with bipyramidal crystals of COD, isolated or grouped in rose-like aggregates.
Ecology & Distribution — The two studied collections have been found growing on coniferous litter of anthropized environments (cemeteries), being difficult to known the ecological requirements of the species. Geographically, those places are located in the ‘Temperate broadleaf and mixed forests’ biome of the Neartic ecozone.
Additional specimens examined (paratypes). USA, Wisconsin, Crawford Co., Prairie du Chien, Prairie du Chien Cemetery, in duff between mature juniper and headstone, 14 Jan. 2006, J.J. Steinke, MICH 72014.
Geastrum subsect. Hungarica J.C. Zamora, subsect. nov. — MycoBank MB810518
Type. Geastrum hungaricum Hollós.
Diagnosis — Exoperidium more or less saccate to arched when hydrated, strongly hygrometric (pseudoparenchymatous layer with thick-walled cells). Mycelial layer encrusting debris, doublelayered. Endoperidial body sessile. Endoperidial surface glabrous and covered by a well-developed mesoperidium, composed by generative hyphae and abundant crystalline aggregates of COM, with scarce bipyramidal crystals of COD. Peristome fibrillose and distinctly delimited.
Geastrum hungaricum Hollós, Math. Termeszettud. Ertes. 19: 507. 1901, ‘Geaster hungaricus‘ — Fig. 9f
Type. Lectotype (designated here, MBT198541): HUNGARY, Nagy-Körös, 3 Dec. 1898, in arenos, Ex Herbario Dr. L. Hollós, Kecskemét, S F16379!
Synoptic description (based on Hollós (1904), Staněk (1958), Sunhede (1989), and studied specimens) — Exoperidium 10–25 mm diam in horizontal position, saccate to arched when hydrated, with 5–12 strongly hygrometric rays. Endoperidial body 2–8 mm diam, whitish to pale greyish brown. Mesoperidium as a thin layer of pruina. Largest mesoperidial crystalline aggregates of COM on the endoperidial surface about 25–30 μm diam, bipyramidal crystals of COD scarce. Peristome fibrillose, distinctly delimited. Stalk absent or indistinct. Basidiospores globose to subglobose, 5.0–6.0 μm diam, with 0.4–0.7 μm high warts, ornamentation verrucose. Pseudoparenchymatous layer very persistent, with clearly thickwalled (> 1.5 μm thick) cells.
Ecology & Distribution — This species mainly grows in open places with scarce trees (Staněk 1958, Sunhede 1989), and most records are known from the ‘Temperate broadleaf and mixed forests’ biome of the Paleartic ecozone. In addition, we have studied one sample from the ‘Mediterranean forests, woodlands and scrub’ biome of the Paleartic ecozone.
Additional specimens examined. CZECH REPUBLIC, Reporyje, u Prahy, slope with Stipa capillata and Gastrosporium simplex, 8 May 1955, Kotlaba, Pouzar & Staněk, herb. Sunhede 5993. – SPAIN, Toledo, Argés, en claro arenoso de encinar, 8 Dec. 2008, J. De Esteban, herb. Zamora 611.
Nomenclatural notes — Although Sunhede (1989) designated a neotype (BP 23244) for this species, original material from herb. Hollós, collected before publication of the name of the species, exists in Stockholm (S F16379). Therefore, the neotype designated by Sunhede (1989) is superseded following Art. 9.19(a), and the collection S F16379 is designated as lectotype.
Geastrum subsect. Quadrifida J.C. Zamora in Zamora et al., Taxon 63, 3: 491. 2014
Key to species in Geastrum subsect. Quadrifida
1. Exoperidium fornicate, with (3–)4–5(–6) rays, margin of rays conspicuously rolled out . . . . . . . . . . . G. quadrifidum
1. Exoperidium not fornicate, with normally more than 4 rays, margin of rays from rolled in to slightly rolled out . . . . . . . 2
2. Basidiomata rather big and stout (exoperidium 45–62 mm diam and endoperidial body 13–21 mm diam); mesoperidium with both abundant generative hyphae and big crystals or crystalline aggregates . . . . . . . . . . . . . . . . . . . . G. kuharii
2. Basidiomata smaller and slender (exoperidium (9–)11–45 (–60) mm diam and endoperidial body 3–14(–15.5) mm diam); mesoperidium with few generative hyphae and abundant or scarce crystals or crystalline aggregates . . . . . . .3
3. Endoperidial surface with a rather sparse mesoperidial crystal cover; largest mesoperidial crystals or crystalline aggregates < 70(–95) μm; stalk mostly dark coloured . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. marginatum
3. Endoperidial surface with a dense mesoperidial crystal cover; largest mesoperidial crystals or crystalline aggregates > (65–)70 μm; stalk light or dark coloured . . . . . . . . . . . . 4
4. Apophysis very well-developed, almost ring-like; stalk 1.5– 2.3 mm high . . . . . . . . . . . . . . . . . . . . . (G. calceum s.l.) 5
4. Apophysis absent to more or less conspicuous, but not ringlike; stalk 0.5–2.0 mm high . . . . . . . . . . . . . . . . . . . . . . .6
5. Stalk slender, 2.0–2.3 mm high, cream coloured to pale brown; basidiospores 4.5–6.0 μm, ornamentation 0.5–0.8 μm high . . . . . . . . . . . . . . . . . . . . . . . . . . G. cf. calceum1
5. Stalk more or less slender or not, 1.5–2.0 mm high, brownish; basidiospores 5.5–6.5 μm, ornamentation (0.5–)0.6– 1.0(–1.2) μm high . . . . . . . . . . . . . . . . . . .G. cf. calceum2
6. Stalk more or less dark coloured, pale brown to rather dark brownish grey; mesoperidium normally with abundant and rather big, rounded, yellowish aggregates of COM; some bipyramidal COD crystals often present, but being rarely the main type; Australasian species . . . G. austrominimum
6. Stalk light-coloured, whitish to cream coloured; mesoperidium mostly with big bipyramidal crystals of COD; crystalline aggregates of COM also often present, but normally much less abundant or inconspicuous; Holartic species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. granulosum
Geastrum austrominimum J.C. Zamora, sp. nov. — MycoBank MB810502; Fig. 6, 11a
Fig. 11.
Morphological characters in Geastrum subsect. Quadrifida (except G. kuharii). a. G. austrominimum (holotype); b. G. cf. calceum1 (MA-Fungi 83761); c. G. cf. calceum2 (UFRN-Fungos 723); d. G. granulosum (Sunhede 7746); e. G. marginatum (MA-Fungi 86669); f. G. quadrifidum (Zamora 590 and MA-Fungi 86671). — a1, b1, c1, d1, e1, f1. Basidiomata habit; a2, b2, c2, d2, e2, f2. detail of the peristome; a3, b3, c3, d3, e3, f3. detail of the stalk and apophysis; a4, b4, c4, d4, e4, f4. mesoperidial crystalline matter on the endoperidial surface: a4 shows crystalline aggregates of COM scales, b4, c4, d4, e4, f4 show bipyramidal crystals of COD; a5, b5, c5, d5, e5, f5. basidiospores. — Scale bars: a1, b1, c1, d1, e1, f1 = 5 mm; a2, b2, c2, d2, e2, f2, a3, b3, c3, d3, e3, f3 = 2 mm; a4, b4, c4, d4, e4, f4 = 10 μm; a5, b5, c5, d5, e5, f5 = 2 μm.
Type. AUSTRALIA, New South Wales, Riverina, Savernake Station, SANDS Plot 8, S35°44' E146°03', 11 June 2001, A. Sloan, S. Reilly, J. Trappe & A. Giachini, Trappe 26378, holotype CANB 748741!, isotype in OSC.
Etymology. The epithet is a combination of the prefix ‘austro-’ and the ending ‘-minimum’, meaning that this species corresponds to the Australasian specimens for which the name G. minimum has been used.
Diagnosis — Exoperidium 17–35 mm diam in horizontal position, arched, with 6–11(–13) not hygrometric rays. Endoperidial body 5–10 mm diam, greyish cream to brownish grey. Mesoperidium as a dense layer of crystalline matter. Largest mesoperidial crystalline aggregates of COM scales 30–105 μm diam, often very abundant and densely covering the endoperidial surface. Largest mesoperidial crystals of COD on the endoperidial surface 20–130(–200) μm diam, bipyramidal, mostly sparse, rarely dominant. Peristome fibrillose, flat to broadly conical, mostly well-delimited. Stalk more or less stout, 0.5–2.0 mm high, normally brownish (paler when old). Basidiospores globose, 4.5–6.5 μm diam, with 0.4–1.0 μm high warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae 5.0–8.5 μm wide. Pseudoparenchymatous layer of thinwalled (mostly ≤ 1 μm thick) cells. Rhizomorphs with rose-like aggregates of bipyramidal crystals of COD.
Macroscopic characteristics — Unexpanded basidiomata not seen. Exoperidium divided in 6–11(–13) more or less equal to unequal rays, 11–32 mm diam apparently, 17–35 mm diam when forced in horizontal position, arched, not hygrometric. Mycelial layer thin to more or less thick, whitish to pale cream, strongly intermixed with debris from the substrate, strongly adhered to the fibrous layer but peeling-off in certain parts. Fibrous layer papyraceous when denuded, whitish to cream coloured. Pseudoparenchymatous layer pale cream to brownish, sometimes superficially cracked, < 0.5 mm thick in dry state, not persisting in old basidiomata. Endoperidial body globose to subglobose, rarely irregular, 5–10 mm diam, pale cream to brownish; endoperidial surface glabrous or almost so. Mesoperidium mainly composed of rather dense and persistent crystalline matter, often with abundant yellowish, rounded crystal aggregates, intermixed with some whitish crystals that are rarely dominant. Peristome fibrillose, of the same colour as the endoperidial surface, mostly flat to broadly conical and distinctly delimited. Stalk present, not particularly slender, 0.5–2 mm high, normally pale brown to rather dark brownish grey, but may be paler in old basidiomata. Apophysis present or absent, the same colour as the endoperidium or slightly darker. Columella intruding about 1/3–1/2 into the glebal mass, with a more or less rounded apex. Mature gleba dark greyish brown.
Microscopic characteristics — Basidia not seen. Basidiospores globose to subglobose, 4.5–6.5 μm diam, brownish to yellowish brown, with 0.4–1.0 μm high brown warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae 5.0–8.5 μm wide, aseptate, normally not branched and more or less straight, thick-walled (2.5–3.5 μm thick), with narrow but mostly visible lumen; tips acute to rounded; surface with or without debris. Endoperidium composed of 2.0–5.5 μm wide, pale yellowish to yellowish brown, aseptate, mostly unbranched, slightly sinuous, strongly intertwined, thick-walled hyphae, lumen mostly visible; protruding hyphae not seen. Peristomal hyphae 4.5–10(–15) μm wide, light brown, aseptate, unbranched except for scarce short branches mostly near the base, thickwalled (1.5–4.0 μm thick), lumen often visible, often sinuous to wavy, narrowing at base and apex, tips mostly rounded, a few acute. Mesoperidium present on the endoperidium and pseudoparenchymatous layer surfaces, composed by rather large, rounded, crystalline aggregates of COM scales, the largest 30–105 μm diam, less abundant bipyramidal crystals of COD, the largest 20–130(–200) μm diam, rarely dominant, and sparse 1.5–3.0 μm wide, hyaline, branched, thin-walled, clamped hyphae. Pseudoparenchymatous layer of thin-walled (≤ 1 μm thick), hyaline to pale yellowish cells, variable in shape and size, about 11–70 × 10–40 μm. Fibrous layer with 3.0–8.0 μm wide, mostly yellowish, aseptate, straight or slightly sinuous, intertwined, mostly unbranched, thick-walled (1.5–3.0 μm thick) hyphae, lumen visible. Mycelial layer double-layered; inner layer consisting of 1.5–3.5 μm wide, strongly glued together, more or less hyaline, branched, thin-walled and clamped hyphae; outer layer with 1.0–4.0 μm wide, hyaline to somewhat yellowish, aseptate, rarely branched, comparatively more or less thick-walled (0.5–2.0 μm thick) hyphae, lumen very narrow and difficult to perceive. Rhizomorphs with rose-like aggregates of bipyramidal crystals of COD.
Ecology & Distribution — The studied specimens came from ‘Temperate grasslands, savannas and shrublands’ and ‘Temperate broadleaf and mixed forests’ biomes of the Australasian ecozone.
Additional specimens examined (paratypes). AUSTRALIA, New South Wales, South Western Plains, Riverina, Savernake Station, SANDS Plot 8, S35°45' E46°01', under Eucalyptus microcarpa, 7 Nov. 2000, J. Trappe 26610, MEL 2276089; Victoria, Neds Corner Station, NW corner of property, VicMuseum pitfall line 004, S34.1137° E141.211°, woodland, 25 Nov. 2011, T. Lebel 2433, MEL 2358014; Victoria, Midlands, Joel Bushland Reserve on south side of Stawell-Landsborough Rd, S37°02' E142°55', Vic. Grid Ref. J 3, 27 July 1996, I.R. McCann, MEL 2103688; Victoria, Murray Mallee, Hattah-Kulkyne National Park, off Mournpall Track, S34°44' E142°21', Vic. Grid Ref. A 45, on dune under Callitris, 7 June 1998, I.R. McCann & T.G. Argall, MEL 2104353; Victoria, Midlands, Kamarooka State Park, East Kamarooka Rd, 500 m N of Boobialla Track, E269185 N5953222, Claridge site 231 - (no EVC), S36°32'18" E144°25'18", 180 m, Vic. Grid Ref. M 30, flat, gravelly-sediment mallee, Eucalyptus behriana, E. froggattii, 4 Aug. 2002, J. Trappe 27826, MEL 2271749; Victoria, Midlands, Black Range, S of Stawell, in Bunjils Cave Reserve, S37°08'13" E142°43'59", Vic. Grid Ref. J 2, near long-leaf Box, 1 Apr. 2001, I.R. McCann & T.G. Argall, MEL 2292062; Victoria, Neds Corner Station, near Snake Lagoon track, S34°08'44.3" E141°23'51.3", blackbox woodland, 30 Nov. 2011, T. Lebel 2372 & A. Pay, MEL 2358047.
Geastrum calceum Lloyd, Mycol. Writings 2: 311. 1907, ‘Geaster calceus’ s.l. — Fig. 6
Type. SOUTH AFRICA, O. Pazschke, C.G. Lloyd Mycological Collection, Smithsonian Institution 57280, BPI 704885 (photo!, probably a syntype).
Synoptic description of G. cf. calceum1 (Fig. 11b) — Exoperidium 41–45 mm diam in horizontal position, arched, with 8–9 not hygrometric rays. Endoperidial body 11–14 mm diam, pale brown to brown. Apophysis almost ring-like. Mesoperidium as a more or less dense layer of crystals. Largest mesoperidial crystals of COD on the endoperidial surface 92–115 μm diam, bipyramidal. Largest mesoperidial crystalline aggregates of COM scales 13–30 μm diam, often very inconspicuous or doubtful. Peristome fibrillose, broadly conical to almost flat, well-delimited. Stalk more or less slender or not, 1.5–2.0 mm high, brownish. Basidiospores globose, 5.5–6.5 μm diam, with (0.5–)0.6–1.0(–1.2) μm high warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae 5.5–6.0 μm wide. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Insufficiently known; the single studied herbarium collection came from an anthropized environment, geographically located in the ‘Tropical and subtropical grasslands, savannas and shrublands’ biome of the Neotropic ecozone.
Additional specimens examined. ARGENTINA, Tucumán, San Miguel de Tucumán, parque Lillo, Nov. 2011, E. Grassi, MA-Fungi 83761.
Synoptic description of G. cf. calceum2 (Fig. 11c) — Exoperidium 38–40 mm diam in horizontal position, arched, with 7–8 not hygrometric rays. Endoperidial body 10–12 mm diam, greyish cream to more or less dark brown. Apophysis almost ringlike. Mesoperidium as a dense layer of crystals. Largest mesoperidial crystals of COD on the endoperidial surface 80–85 μm diam, bipyramidal. Largest mesoperidial crystalline aggregates of COM scales 12–15 μm diam, often very inconspicuous or doubtful. Peristome fibrillose, broadly conical to almost flat, well-delimited. Stalk slender, 2.0–2.3 mm high, cream coloured to pale brown. Basidiospores globose, 4.5–6.0 μm diam, with 0.5–0.8 μm high warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae 7.0–8.0 μm wide. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Insufficiently known; studied specimens came from ‘Tropical and subtropical moist broadleaf forests’ biome of the Neotropic ecozone.
Additional specimens examined. BRAZIL, Rio Grande do Norte, Natal, Tricha da Geologia, P. Dunas, 14 Apr. 2010, M.M.B. Barbosa, B. Tomio, T. Lockwood & I.G. Baseia, UFRN-Fungos 723. – COSTA RICA, Guanacaste, Parque Nacional Santa Rosa, Bosque de San Emilio, 300 m, 15 Apr. 2003, M. Mata 1279, MA-Fungi 65435 (duplo INB 3721248).
Additional remarks — Although requested on loan, type material of G. calceum was not available for detailed morphological study, which prevented us to make a proper synoptic description because of the absence of important morphological characteristics, such as micromorphological data, in the protologue.
Geastrum granulosum Fuckel, Jahrb. Nassauischen Vereins Naturk. 15: 41. 1860, ‘Geaster granulosus’ — Fig. 6, 11d
Type. Lectotype (designated here, MBT198518): GERMANY, c. Budenheim, Fuckel 317, UPS F-127434!
= G. queletii Hazsl., Verh. Bot. Vereins Prov. Brandenburg 24: 136. 1883, ‘Geaster Quéletii’. — Type: Lectotype (designated here, MBT198519): FRANCE, Jura, Quélet, S F16394!
Synoptic description (based on Staněk (1958, as G. minimum var. minimum) and studied specimens) — Exoperidium (9–)11– 42(–60) mm diam in horizontal position, arched, with 5–13(–14) not hygrometric rays. Endoperidial body 3–12(–15.5) mm diam, cream coloured, greyish or brownish, less frequently whitish. Mesoperidium as a dense layer of crystalline matter. Largest mesoperidial crystals of COD on the endoperidial surface 65–165 μm diam, bipyramidal, normally very abundant. Largest mesoperidial crystalline aggregates of COM scales (7–)10–62(–120) μm diam, often present but inconspicuous, sometimes abundant. Peristome fibrillose, flat or conical, mostly distinctly delimited. Stalk more or less stout, (0.3–)0.5–2.0 mm high, whitish to cream coloured. Basidiospores globose, 4.5– 6.5(–7.5) μm diam, with (0.3–)0.4–0.8(–1.0) μm high warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae 4.5–9.5 μm wide. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Confirmed collections of this species came from calcareous soils on both open places and forests, of a wide range of biomes (‘Boreal forests/taiga’, ‘Temperate conifer forests’, ‘Temperate broadleaf and mixed forests’, and ‘Mediterranean forests, woodlands and scrub‘) of the Neartic and Paleartic ecozones.
Additional specimens examined. GERMANY, Oestrich, Nassau, c. Budenheim, in pinetis, Fuckel, herbier Barbey-Boissier 2138, UPS; c. Budenheim, in pinetis, 1265, UPS F-127433. – SPAIN, Madrid, Alcalá de Henares, 10 Dec. 1993, G. Manteiga, MA-Fungi 32165; Madrid, Alcalá de Henares, cerro del Viso, bajo Pinus halepensis, terreno básico, 12 Nov. 2011, R. Suárez Rico, herb. Zamora 600; Madrid, Alcalá de Henares, bajo Pinus halepensis, suelo calizo superficialmente quemado, 20 Nov. 2011, J. Hernanz, B. Zamora & J.C. Zamora, herb. Zamora 550; Madrid, Arganda del Rey, dehesa ‘El Carrascal’, 30 Nov. 2003, L. Rubio, MA-Fungi 59067; ibid., bajo conífera indeterminada, 18 Nov. 2006, L. Penelas, herb. Zamora 191; Madrid, Arganda del Rey, bajo Quercus coccifera, 30 Nov. 2005, L. Rubio, MA-Fungi 69175; Madrid, Cerro de los Ángeles, en pinar de Pinus halepensis, 8 Dec. 1991, A. Guerra, MA-Fungi 28118; Murcia, Fuente del Obispo, sierra de la Fuensanta (sierra Carrascoy), 30SXG6497, 500 m, entre agujas de Pinus halepensis, 9 Nov. 1978, M. Honrubia, MA-Fungi 42282 (ex MUB 1653); ibid., pinar de Pinus halepensis, en fisuras de rocas, 1 Mar. 1980, X. Llimona, MHG 2975, MA-Fungi 42283 (ex MUB 1656); ibid., pinar aclarado de Pinus halepensis, 14 Nov. 1978, M. Honrubia, MHG 343, MA-Fungi 42284 (ex MUB 1654); ibid., pinar de Pinus halepensis, 22 May 1978, M. Honrubia, MHG 311, MAFungi 42287 (ex MUB 1658); ibid., pinar de Pinus halepensis, 14 Nov. 1978, M. Honrubia, MHG 343, MA-Fungi 43290 (ex MUB 343); Soria, cañón del río Lobos, bajo Pinus sp. y Juniperus thurifera, 9 Nov. 1992, T. Almaraz, MA-Fungi 32393; Tarragona, La Cenia, 10 Nov. 1973, J.M. Alemany, MAFungi 42280 (ex MUB 1650); Teruel, puerto de Alcalá de la Selva, 28 Oct. 1993, F.D. Calonge, MA-Fungi 32164; Teruel, Bezas, campo descubierto, 27 Oct. 1993, F.D. Calonge, MA-Fungi 32163; Teruel, Formiche, 24 Oct. 1990, F.D. Calonge, MA-Fungi 31162; Teruel, Linares de Mora, 25 Oct. 1995, bajo Pinus sylvestris, F.D. Calonge, MA-Fungi 34054; Teruel, Valdelinares, bajo Pinus sylvestris, 28 Oct. 1994, F.D. Calonge, MA-Fungi 33261; Valladolid, Portillo, bajo Pinus pinea, 20 Dec. 1999, A. García, MA-Fungi 47907. – Sweden, Öland, Gårdby parish, N. Gårdby, open sandy grazed and trampled ground, with i.a. Veronica spicata, near forest border (Pinus sylvestris), 28 Aug. 2012, J.C. Zamora & S.I. Sunhede, herb. Sunhede 7746. – USA, Arizona, Coconino Co., Walnut Canyon National Monument, Pinus edulis, 14 Oct. 1986, J. States AEF 810, MICH 28210; ibid., in open area around debris, Pinus edulis and Juniperus, 17 Oct. 1986, J. States AEF 518, MICH 28119a; Wisconsin, Waukesha Co., NW corner of Hwy E and Hwy 99, very stony, specimens in needle duff around two juniper trees near top of hill, 25 Oct. 1998, J.J. Steinke, MICH 72010.
Nomenclatural notes — At UPS herbarium there are three herbarium specimens (UPS F-127433 (1265), UPS F-127434 (Fuckel 317), and Herbier Barbey-Boissier 2138) collected by Fuckel and fitting the data of the protologue. The collection UPS F-127433 contains three basidiomata, all of them wellconserved and two of them with the pseudoparenchymatous layer in good condition, glued to a sheet of paper in different positions, and clearly showing both big crystals on endoperidial surfaces, and a whitish stalk. The collection UPS F-127434 consists of a single, well-conserved basidioma with big crystals on the endoperidial surface, being the stalk hidden by the pressing procedure, but if the endoperidial body is carefully lifted, a short, whitish stalk can be seen. This collection was additionally marked by C.G. Lloyd as “This is a type but the species = G. minimus”, and the number 317 is the one that Fuckel (1860) used for enumerating the species in the protologue. Finally, the collection Herbier Barbey-Boissier 2138 contains another three basidiomata, one of them rather young and the other two somewhat old, showing big crystals on endoperidial surfaces and whitish stalks as well. All these specimens are in full agreement with the concept of the species followed here. One of these collections can be selected as lectotype and, since Lloyd considered the collection UPS F-127434 as ‘type’, and the number 317 is the one used by Fuckel in the protologue of the species, we designate this collection as the lectotype for the species. Geastrum queletii was briefly described by Hazslinszky (1883) based on material collected by Quélet (1873), who named it as ‘Geaster umbilicatus’. There is one specimen at the Stockholm herbarium marked as ‘typus’ (S F16394) by a latter hand than that of Hazslinsky, and in the original label it is marked ‘Geaster Quéleti Hasz.’. It consists of a single, well-preserved basidioma, which shows big crystals on the endoperidial surface and a whitish stalk, collected from Jura by Quélet. This specimen has been designated as lectotype and, in agreement with our concept of G. granulosum, it is considered as a heterotypic synonym.
Geastrum kuharii J.C. Zamora, sp. nov. — MycoBank MB810503; Fig. 4, 10e
Type. ARGENTINA, Buenos Aires, Partido de Berazategui, Pereyra, Pereyra Iraola park, Pinus sp., 16 June 2012, F. Kuhar, V. Castiglia & J.C. Zamora, holotype MA-Fungi 83795!, isotypes in AH 45202! and UPS!
Etymology. The specific epithet is dedicated to Francisco Kuhar, Argentinian mycologist expert in Geastrum, who helped collecting the type specimen.
Diagnosis — Exoperidium 45–62 mm diam in horizontal position, arched, with 8–11 not hygrometric rays. Endoperidial body 13–21 mm diam, greyish cream to brownish grey. Mesoperidium rather well-developed, as a mealy cover with big crystals. Largest mesoperidial crystals of COD on the endoperidial surface 75–130 μm diam, bipyramidal. Largest mesoperidial crystalline aggregates of COM scales 70–95 μm diam, sometimes not very conspicuous. Peristome fibrillose, flat, well-delimited. Stalk stout 1.5–2.5 mm high, pale brown to brown. Basidiospores globose, 4.5–5.5(–6.0) μm diam, with 0.4–0.7(–0.8) μm high warts. Broadest capillitial hyphae 6.0–7.0 μm wide, ornamentation verrucose to irregularly pilate. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells. Rhizomorphs with bipyramidal crystals of COD, grouped in rose-like aggregates.
Macroscopic characteristics — Unexpanded basidiomata 20–33 mm diam, subglobose to depressed, with a rounded apex or a flat umbo. Exoperidium splitting in 8–11 more or less equal to unequal rays, 30–55 mm diam apparently, 45–62 mm diam when forced in horizontal position, arched, not hygrometric. Mycelial layer rather thick, whitish to pale cream, sometimes with pinkish shades when fresh, strongly intermixed with debris from the substrate, more or less strongly adhered to the fibrous layer but often peeling-off in some parts. Fibrous layer more or less coriaceous when denuded, pale-cream to cream, in fresh sometimes turning pinkish when damaged. Pseudoparenchymatous layer whitish at first, surface cream to greyish, normally superficially cracked, < 0.5 mm thick in dry state, about 2.0–4.0 mm thick when fresh, not very persistent. Endoperidial body globose to subglobose, 13–21 mm diam, endoperidial surface glabrous, brownish. Mesoperidium conspicuous, with both abundant generative hyphae and crystalline matter. Peristome fibrillose, often slightly darker than the endoperidial surface, flat, thickened, and distinctly delimited. Stalk stout, 1.5–2.5 mm high, pale brown to brown. Apophysis present, well-developed or not, sometimes almost ring-like, with the same colour as the endoperidium. Columella intruding about 1/2 into the glebal mass, stout, with a rounded apex. Mature gleba dark brown.
Microscopic characteristics — Basidia narrowly ellipsoid to subcylindric or more or less lageniform, 15–26 × 4.5–6.5 μm, with (4–)5–8 short sterigmata. Basidiospores globose to subglobose, 4.5–5.5(–6.0) μm diam, brownish, with 0.4–0.7(–0.8) μm high brown warts, verrucose to irregularly pilate ornamentation. Broadest capillitial hyphae 6–7 μm wide, aseptate, very rarely branched, normally straight, thick-walled (1.5–3 μm thick), with narrow lumen, mostly visible; tips acute to rounded; surface often covered with debris. Endoperidium composed of 1.5–6.0 μm wide, pale yellowish to yellowish brown, aseptate, mostly unbranched, straight to slightly sinuous, strongly intertwined, thick-walled hyphae, lumen visible; protruding hyphae not seen. Peristomal hyphae 5.0–10.0 μm wide, brownish, aseptate, mostly unbranched, thick-walled (1.0–3.0 μm thick), lumen normally wide, often sinuous, narrowing at base and apex, tips often acute. Mesoperidium present on the endoperidium and pseudoparenchymatous layer surfaces, consisting of rather large bipyramidal crystals of COD, the largest 70–135 μm diam, and abundant 1.5–4.0 μm wide, hyaline to pale brownish, branched, thin-walled, clamped hyphae; rounded crystalline aggregates of COM scales up to 70–95 μm diam, sometimes inconspicuous. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick), hyaline cells, variable in shape and size, about 15–80 × 10–42 μm diam. Fibrous layer with 1.5–6.0 μm wide, hyaline to very pale yellowish, aseptate, straight or slightly sinuous, intertwined, mostly unbranched, comparatively thick-walled (0.7–2.5 μm thick) hyphae, lumen visible. Mycelial layer double-layered; inner layer consisting of 1.5–3.0 μm wide, strongly glued together, more or less hyaline, branched, thin-walled and clamped hyphae; outer layer with 1.0–4.0 μm wide, hyaline to somewhat yellowish, aseptate, rarely branched, comparatively thick-walled (0.5–2.0 μm thick) hyphae, lumen very narrow and difficult to perceive. Rhizomorphs with bipyramidal crystals of COD, often grouped in rose-like aggregates.
Ecology & Distribution — Only known from humic soils of the ‘Temperate grasslands, savannas and shrublands’ biome of the Neotropic ecozone.
Additional specimens examined (paratypes). ARGENTINA, Buenos Aires, Partido de Berazategui, Pereyra, Pereyra Iraola park, Pinus sp., 16 June 2012, F. Kuhar, V. Castiglia & J.C. Zamora, MA-Fungi 86914; Entre Ríos, Colón, Ubajay, marginal forest, 2 June 2012, S. Suaza & J.C. Zamora, MAFungi 86913.
Geastrum marginatum Vittad., Monographia Lycoperdineorum: 20. 1842, ‘Geaster marginatus‘ — Fig. 6, 11e
Type. Lectotype (designated here, MBT198520): f. 6 of pl. I in Vittadini (1842). Epitype (designated here, MBT198521) that supports the lectotype cited above: Spain, Madrid, El Pardo-Fuencarral, encinar, 6 Dec. 2000, J.C. Campos y familia, MA-Fungi 48129!
= Geastrum cesatii Rabenh., Bot. Zeitung (Berlin) 9, 36: 628. 1851, ‘Geaster Cesatii’. — Type: Unknown (but see nomenclatural notes below).
= Geastrum minimum var. fumosicollum V.J. Staněk in Pilát, Flora ČSR B-1: 435, 786. 1958. — Type: CZECH REPUBLIC, Bohemia, Velká Chuchle ap. Praha, 18-IX-1954, E. Wichanský, holotype PRM 842884! Various paratypes were also cited by Staněk (1958).
? = Geastrum juniperinum T. Macbr., Mycologia 4, 2: 85. 1912, ‘Geaster juniperinus’. — Type: Unknown. In absence of original specimens, f. 1 of pl. LXII in Macbride (1912) can be selected as lectotype.
Synoptic description (based on Staněk (1958, as G. minimum var. fumosicollum) and studied specimens) — Exoperidium 13–40 mm diam in horizontal position, arched, with 5–13(–14) not hygrometric rays. Endoperidial body 3–14 mm diam, greyish to greyish brown, rarely whitish or very dark. Mesoperidium as a thin layer of sparse pruina. Largest mesoperidial crystals of COD on the endoperidial surface (5–)7.5–70(–95) μm diam, bipyramidal, often rather scarce. Largest mesoperidial crystalline aggregates of COM scales 5–25(–40) μm diam, normally inconspicuous, rarely abundant. Peristome fibrillose, flat or conical, mostly distinctly delimited. Stalk more or less stout, (0.3–)0.5–2.0 mm high, mostly brownish, to greyish brown or even blackish, less frequently pale coloured. Basidiospores globose, 4.5–7.0(–8.0) μm diam, with (0.3–)0.4–0.8(–1.0) μm high warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae 4.5–9.0 μm wide. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Confirmed specimens came from mostly sandy, siliceous or slightly calcareous soils, from both open places and forests, of several biomes (‘Temperate conifer forests’, ‘Temperate broadleaf and mixed forests’, and ‘Mediterranean forests, woodlands and scrub‘) of the Neartic and Paleartic ecozones.
Additional specimens examined. ITALY, Ne’ luoghi arenosi e scarsi di erbe lungo gli argini del fume Sesia presso Vercelli, Dec. 1859–60, Cesati, Erbario Crittogamico Italiano ser. I, B 70 0014089; ibid., ex herb. Sydow, S; Sesia presso Vercelli, 1860, Cesati, S. – SPAIN, Ávila, camino de Piedralaves a La Adrada, 4 Dec. 1984, F.D. Calonge, MA-Fungi 8112; Huesca, Javierregai, 676 m, encinar con jaras, suelo arcilloso, 28 Oct. 2005, F. Prieto, Á. González & C. Diego, herb. Zamora 108; Canary Islands, La Gomera, pantano de Arure-Acardece, 882 m, en zona arbustiva, 26 Nov. 2012, J. Fernández, M. Oyarzabal, F. Hidalgo & R. Martínez, ERRO 2012112609; Jaén, Baños de la Encina, junto al río Aliseda, 28 Nov. 1993, F.D. Calonge, MA-Fungi 32395; Jaén, Venta de los Santos, 12 Oct. 2000, J.C. Zamora, MA-Fungi 48128; Madrid, Majadahonda, 3 Dec. 1989, F.D. Calonge, MA-Fungi 31530; Madrid, El Pardo, entrada por El Goloso, en suelo arenoso de encinar, 11 Nov. 1978, E. Álvarez, MA-Fungi 724; Madrid, El Pardo, bajo encina, 4 Feb. 1979, E. Álvarez, MA-Fungi 725; Salamanca, Valdelosa, pradera al borde de camino, 10 Oct. 1993, J. Lozano, MA-Fungi 32394; Sevilla, Puebla de los Infantes, Los Cerrillares, 8 Dec. 2008, M.Á. Ribes, herb. Ribes 081208-03; Teruel, Albarracín, en encinar, 26 Oct. 1988, F.D. Calonge, MA-Fungi 21695. – Sweden, Gotland, Holmhällar (close to the hotel), 28 Sept. 2011, open area on sandy soil, among lichens, mosses and grasses, not far away from Pinus sylvestris, J.C. Zamora, M. Jeppson & M. Lathi, MA-Fungi 86669. – USA, Arizona, Coconino Co., Walnut Canyon National Monument, N35°10" W111°30.26", Juniperus, 12 Oct. 1986, J. States AEF 528, MICH 28120; Arizona, Coconico Co., Walnut Canyon National Monument, in open area around debris, Pinus edulis and Juniperus, 17 Oct. 1986, J. States AEF 518, MICH 28119b.
Nomenclatural notes — There is one collection at herbarium K marked by a latter hand than that of Vittadini (1842) as “Geaster marginatum Vittad. Isotypus. Overseas, sine loc.; on soil; ex herb. M. J. Berkeley; Herbarium Mycologicum Berkeleyanum, presented by the Rev. M.J. Berkeley, 1879; K(M) 169190”, which consists of three more or less well-preserved basidiomata, in agreement with the protologue and with the concept of the species presented here. However, whether it is part of the original material studied by Vittadini or not is difficult to say with certainty, due to the absence of ecological and geographical data on the herbarium label. The description and the iconography from Vittadini (1842) suggest that the concept he used may be the same as considered here, but they are not detailed enough to clearly distinguish this species from the morphologically close G. granulosum, because crystals on the endoperidial surface and the stalk colour are not mentioned. For this reason, although f. VI of t. I in Vittadini (1842) is part of the protologue and has been designated here as lectotype, in absence of unambiguous original material, an epitype should also be chosen in order to fix the interpretation of this species. Therefore, we have designated as epitype one sequenced specimen from the Mediterranean basin, collected in sandy, siliceous soil, which consists of six well-developed and typical basidiomata. Geastrum cesatii (Rabenhorst 1851) was described from specimens collected by Cesati in Piedmont (near Vercelli). We have not been able to trace any original material, but there are two specimens in the Stockholm herbarium (S), and one in the Berlin herbarium (B), collected by Cesati near Vercelli after Rabenhorst’s (1851) publication, being part of the Erbario Crittogamico Italiano serie I, n. 590, in agreement with the protologue and with the concept of G. marginatum followed here. Thus, the name is considered as a heterotypic synonym of G. marginatum. In absence of original material, any of these collections may serve as a good neotype for the name. Geastrum minimum var. fumosicollum (Staněk 1958) is also considered as a heterotypic synonym, since morphological data agree with those of typical basidiomata of G. marginatum, and molecular data place the holotype within the strongly supported G. marginatum clade (Fig. 1). Geastrum juniperinum (Macbride 1912) was not described in detail, but according to the iconography of the protologue it could be possibly another heterotypic synonym. Since original specimens have not been studied by us yet, and considering that some species in G. subsect. Quadrifida are difficult to be distinguished, we prefer to indicate such synonymy with an expression of doubt.
Geastrum quadrifidum Pers., Neues Mag. Bot. 1: 86. 1794; Pers., Syn. Meth. Fung.: 133. 1801. — Fig. 3, 11f
Type. Lectotype (designated here, MBT198522): f. 1 of pl. XXXVII in Schmidel (1793).
= Geastrum coronatum (Scop.) J. Schröt. in Cohn, Krypt.-Fl. Schlesien 3–1, 6: 702. 1889, ‘Geaster coronatus’, nom. illeg. (Art. 53.1).
≡ Lycoperdon coronatum Scop., Fl. Carniol. 2: 490. 1772. — Type: Lectotype (designated here, MBT198523): f. 3 of pl. CLXXXIII in Schaeffer (1763).
= Geastrum quadrifidum var. minus Pers., Syn. Meth. Fung.: 133. 1801.
≡ Geastrum minus (Pers.) G. Cunn., Proc. Linn. Soc. New South Wales 51: 81. 1926, ‘Geaster’. — Type: Lectotype (designated here, MBT198524): f. 2 of pl. XXXVII in Schmidel (1793).
= Geastrum quadrifidum var. sabulosum V.J. Staněk in Pilát, Flora ČSR B-1: 439, 794. 1958. — Type: SLOVAKIA, Píscové přesypy mladém boru u Malacek, 1 May 1954, V.J. Staňkovi, holotype PRM (photo!); Kúty, loco arenoso, 17 Nov. 1955, F. Kotlaba, paratype PRM 842888!
Synoptic description (based on Sunhede (1989) and studied specimens) — Exoperidium (15–)17–50(–90) mm diam in horizontal position, arched, with (3–)4–5(–6) not hygrometric rays. Endoperidial body (3.5–)5–12(–16) mm diam, pale cream to rather dark greyish brown. Mesoperidium as a thin layer of pruina. Largest mesoperidial crystals of COD on the endoperidial surface (10–)15–50(–60) μm diam, bipyramidal, abundant. Mesoperidial crystalline aggregates of COM scales normally very scarce or inconspicuous, about 5–25(–30) μm diam. Peristome fibrillose, conical to papillate, rarely flat, mostly sharply delimited. Stalk often stout, (0.3–)1.0–2.0(–2.5) mm high, whitish to brownish grey. Basidiospores globose, (4.5–)5.0– 6.0(–6.5) μm diam, with (0.3–)0.4–0.8 μm high warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae (4.0–)4.5–9.5 μm wide. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Mainly growing in calcareous soils of ‘Boreal forests/taiga’, ‘Temperate conifer forests’, and ‘Temperate broadleaf and mixed forests’ biomes of the Neartic and Paleartic ecozones, but also present in the ‘Mediterranean forests, woodlands and scrub’ biome (Sunhede 1989, Calonge 1998, Bates 2004).
Additional specimens examined. SPAIN, Cuenca, Almodóvar del Pinar, bosque de Q. rotundifolia, Q. coccifera, Pinus pinaster y algún Q. faginea, suelo calizo, 25 Oct. 2008, J.F. Mateo, F. Gracia & J.C. Campos, herb. Zamora 300; Huesca, Canfranc, bajo Picea abies, 14 Oct. 2006, M. Chiaffi, herb. Zamora 170; Huesca, Bielsa, valle de Pineta, bajo Abies alba, 13 Oct. 2012, J. Hernanz, herb. Zamora 561; Madrid, Guadalix de la Sierra, ‘El Montecillo’, bosque de Quercus rotundifolia y Q. faginea, suelo calizo, 5 Nov. 2011, A. Díaz, herb. Zamora 590; Orense, O Barco de Valdeorras, pinar Vegamolinos, bajo Pinus sp., entre musgos, 11 Jan. 2006, C. Ruiz, herb. Zamora 139. – Sweden, Gotland, Stenkyrka parish, Snipklint, Picea abies, Pinus sylvestris forest, with Juniperus communis on limestone, on needle litter under dense spruces, 7 Apr. 1968, D. Lowgren, B. Lowgren & S. Sunhede, herb. Sunhede 97a; Uppland, Älkarleby, Billuddens naturreservat, under Pinus sylvestris, Picea abies, and Betula pendula, on sandy, calcareous soil, 15 Oct. 2011, I. Olariaga & J.C. Zamora, MA-Fungi 86671. – USA, California, Siskiyou Co., 2 mi E of McCloud, in duff in Pseudotsuga menziesii, Abies concolor, Quercus and Pinus forest, 25 Aug. 1990, Richter D. DR90-05, MICH 39032; Colorado, Boulder Co., vicinity Castle Rock in Middle Boulder Creek Canyon, 1.5 mi northeast of Tungsten, N39°59' W105°27', c. 7900 ft., 11 Sept. 1970, R. Fogel F2078, MICH 72512; Minnesota, Clearwater Co., Itasca St. Pk., 26 Sept. 1970, K.E. Harrison, MICH 72511.
Nomenclatural notes — As noted by Sunhede (1989), the specimen designated as type by Eyndhoven (1937) should be considered as a neotype. Therefore, although in perfect agreement with the current concept of this species, it is to be superseded according to Art. 9.19(a) because original material (pl. XXXVII in Schmidel (1793)) exists. Protologues and original material of G. coronatum (Scop.) J. Schröt. (nom. illeg.), G. quadrifidum var. minus, and G. quadrifidum var. sabulosum agree with the concept of the species followed here, and are considered heterotypic synonyms.
Geastrum subsect. Sulcostomata V.J. Staněk in Pilát, Flora ČSR B1, Gasteromycetes: 781. 1958, nom. inval. (Art. 22.2)
≡ Geastrum subg. Pectinata Dörfelt & Müll.-Uri in Dörfelt, Die Erdsterne: Geastraceae und Astraeaceae: 17. 1985. — Type: Geastrum pectinatum Pers. (Fig. 1E).
= Geastrum sect. Striata De Toni, Rev. Mycol. 9, 34: 64. 1887, ‘Striati’. — Type: Geastrum striatum DC.
Nomenclatural notes — This name is currently not validly published according to Art. 22.2 (McNeill et al. 2012) but, as noted in Zamora et al. (2014) by the nomenclature editor of the journal Taxon, the name is used pending potential amendments to Art. 22.2.
Key to species in Geastrum subsect. Sulcostomata
1. Apophysis very well-developed, solid, ring-like . . . . . . . . . 2
1. Apophysis different or absent; if present, then as a fold of the endoperidium . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
2. Apophysis with a sharp and acute edge, basidiospores 5.0– 6.0 μm diam . . . . . . . . . . . . . . . . . . . . . . . . . . . G. striatum
2. Apophysis with a rounded edge, basidiospores 4.5–5.0 μm diam . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. aff. Striatum
3. Stalk (2.5–)3–13 mm high, frequently slender, length/breath = 0.7–7.5(–8.6); species preferring humid to subhumid habitats; basidiospore ornamentation clearly baculate-pilate . 4
3. Stalk 0.5–2.5(–3) mm high, mostly stout, length/breath = 0.5– 1(–1.5); species preferring subhumid to semixeric habitats; basidiospore ornamentation verrucose to irregularly pilate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .8
4. Largest mesoperidial crystals 20–55 μm diam; Australasian species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. tenuipes
4. Mesoperidial crystals up to 15(–32) μm diam; not Australasian species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5
5. Basidiospores (4.5–)5.0–6.5 μm diam; stalk 0.7–2.5 mm width . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
5. Basidiospores 5.5–7.5(–8.5) μm diam; stalk (1.6–)2.0– 8.0(–9.0) mm width . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7
6. Broadest capillitial hyphae 6.0–8.0 μm diam; Pantropical species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. plicatum
6. Broadest capillitial hyphae up to 10.5 μm diam; Oceanian species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. biplicatum
7. Broadest capillitial hyphae (5.5–)6.0–9.0(–9.5) μm diam; stalk relatively stout, length/breath = 0.6–2.3, light or dark coloured; peristome with (17–)22–52(–60) folds; endoperidium mostly light coloured, from cream to brownish grey, never blackish; Mediterranean and Macaronesian species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. meridionale
7. Broadest capillitial hyphae (8.0–)9.0–13.0(–15.0) μm diam; stalk mostly slender, length/breath = 1.7–4.0, mostly light coloured; peristome with 11–35 folds; endoperidium very variable in colour, often dark, even blackish; Temperate to Boreal species . . . . . . . . . . . . . . . . . . . . . . .G. pectinatum
8. Basidiospores 4.0–5.5 μm diam, brownish, ornamentation 0.3–0.6 μm high; broadest capillitial hyphae 4.5–6.0 μm diam; glebal mass brownish to dark brown; basidiomata rather slender, not reminiscent G. striatum, endoperidial body 3.5– 6.5 mm diam, exoperidium 12–24 mm diam . G. papinuttii
8. Basidiospores (4.5–)5.0–7.0 μm diam, dark brown to blackish, ornamentation (0.4–)0.5–1.0(–1.2) μm high; broadest capillitial hyphae 5.5–9.0 μm diam; gleba dark brown to blackish; basidiomata comparatively stout, often strongly reminiscent G. striatum, endoperidial body 5–16 mm diam, exoperidium (13–)18–47 mm diam . . . . . . . . . . . . . . . . . . . . . . . . . . .9
9. Columella mostly with a persistent and pointed apex; South American species . . . . . . . . . . . . . . . . . . . G. glaucescens
9. Columella mostly with a deciduous, often rounded but sometimes pointed, apex; South European species . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . G. parvistriatum
Geastrum biplicatum Berk. & M.A. Curtis, Proc. Amer. Acad. Arts 4: 124. 1860, ‘Geaster biplicatus’ — Fig. 12a
Fig. 12.
Morphological characters in Geastrum subsect. Sulcostomata pro parte (G. pectinatum group). a. G. biplicatum (lectotype); b. G. meridionale (holotype); c. G. pectinatum (epitype); d. G. cf. plicatum (MA-Fungi 83774); e. G. tenuipes (CANB 738350). — a1, b1, c1, d1, e1. Basidiomata habit; a2, b2, c2, d2, e2. detail of the peristome; a3, b3, c3, d3, e3. detail of the stalk and apophysis; a4, b4, c4, d4, e4. mesoperidial crystals of COD on the endoperidial surface; a5, b5, c5, d5, e5. basidiospores. — Scale bars: a1, b1, c1, d1, e1 = 5 mm; a2, b2, c2, d2, e2, a3, b3, c3, d3, e3 = 2 mm; a4, b4, c4, d4, e4 = 10 μm; a5, b5, c5, d5, e5 = 2 μm.
Type. Lectotype (designated here, MBT198525): JAPAN, Bonin Islands, on the ground, US N Pacific Expedition Exp. (ex. herb. Hooker), K(M) 180394! (probably one of various syntypes).
Synoptic description (based on the type specimen only) — Exoperidium 34 mm diam in horizontal position, with 7 nonhygrometric rays. Endoperidial body 11 mm diam, dark brown. Mesoperidium well-formed, powdery. Largest mesoperidial crystals of COD on the endoperidial surface about 15 μm diam, bipyramidal. Peristome sulcate, more or less distinctly delimited, with about 19 folds. Stalk slender, about 2.0 mm high and 1.0 mm wide, brownish. Base of the endoperidial body more or less sulcate. Basidiospores globose, (4.5–)5.0–6.0 μm diam, with 0.5–1.0 μm high warts, ornamentation baculate-pilate. Broadest capillitial hyphae 10.5 μm diam. Cells of the pseudoparenchymatous layer deteriorated and thus not studied, expectedly thin-walled.
Ecology & Distribution — Insufficiently known; the type specimen was collected in the Bonin Islands (Berkeley & Curtis 1860), which are located in the ‘Tropical and subtropical moist broadleaf forests’ biome of the Oceania ecozone.
Additional remarks — Specimens included in the phylogenetic tree as ‘G. cf. biplicatum’ were not available for morphological study even if requested on loan (see Discussion).
Geastrum glaucescens Speg., Anales Mus. Nac. Hist. Nat. Buenos Aires 23: 14. 1912, ‘Geaster’ — Fig. 7, 13a
Fig. 13.
Morphological characters in Geastrum subsect. Sulcostomata pro parte (G. striatum s.l. and G. glaucescens group). a. G. glaucescens (MA-Fungi 83762); b. G. parvistriatum (Zamora 580); c. G. papinuttii (holotype); d. G. striatum (Zamora 242); e. G. aff. striatum (AH 18521). — a1, b1, c1, d1, e1. Basidiomata habit; a2, b2. median section of the endoperidial body showing the columella with a pointed and persistent apex (a2) or a more rounded and evanescent apex (b2); c2, d2, e2. detail of the peristome; c3, d3, e3. detail of the stalk an apophysis; a3, b3, c4, d4, e4. mesoperidial crystals of COD on the endoperidial surface; a4, b4, c5, d5, e5. basidiospores. — Scale bars: a1, b1, c1, d1, e1 = 5 mm; a2, b2, c2, d2, e2, c3, d3, e3 = 2 mm; a3, b3, c4, d4, e4 = 10 μm; a4, b4, c5, d5, e5 = 2 μm.
Type. ARGENTINA, Buenos Aires, La Plata, Spegazzini, Sept. 1894, holotype LPS 15860!
Synoptic description — Exoperidium 18–45 mm diam in horizontal position, arched, divided in 6–11 non-hygrometric rays. Endoperidial body 5–16 mm diam, mostly dark brown to blackish. Columella with a persistent and pointed apex, rarely rounded. Mesoperidium very abundant, powdery. Largest mesoperidial crystals of COD on the endoperidial surface 6.0–15 μm diam, bipyramidal. Peristome sulcate, distinctly delimited or not, with 11–25 folds. Stalk 0.5–2.5 mm high, mostly brownish to dark brown. Basidiospores globose, 5.0–7.0(–7.5) μm diam, with 0.5–1.0(–1.2) μm high warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae 6.0–9.0 μm diam. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — This species grows mostly under mesquite (Prosopis spp.), and has been found in ‘Temperate grasslands, savannas and shrublands’ and ‘Tropical and subtropical grasslands, savannas and shrublands’ biomes of the Neotropic ecozone. Currently only known from Argentina (Soto & Wright 2000, Kuhar et al. 2013).
Additional specimens examined. ARGENTINA, Catamarca, San Fernando del Valle de Catamarca, Prosopis sp., 10 Apr. 2012, L. Papinutti & J.C. Zamora, MA-Fungi 83763; La Rioja, Castro Barros, Anjullón, Prosopis sp., 8 Apr. 2012, L. Papinutti & J.C. Zamora, MA-Fungi 83762; ibid., MA-Fungi 86906; ibid., MA-Fungi 86907; ibid., MA-Fungi 86908; ibid., MA-Fungi 86909; ibid., MA-Fungi 86910; La Rioja, Santa Cruz, S28°28'35.11" W67°41'54.45", on abundant Prosopis sp. leaf litter, 27 Mar. 2008, L. Papinutti & G. Rolón, BAFC 51940.
Geastrum meridionale J.C. Zamora, sp. nov. — MycoBank MB810504; Fig. 12b
Type. SPAIN, Madrid, Ciudad Universitaria, E.T.S.I. de Montes, under Calocedrus decurrens, 17 Oct. 2007, J.C. Zamora 252, holotype MA-Fungi 87325!, isotypes in AH 45203! and UPS!
Etymology. The epithet refers to the southern distribution of the species in Europe, especially when compared with the mostly central and northern distribution of its closest relative, G. pectinatum.
Diagnosis — Exoperidium 45–125 mm diam in horizontal position, divided in 5–12 non-hygrometric rays. Endoperidial body 12–33 mm diam, cream to brownish grey, rarely dark brown. Mesoperidium very abundant, powdery. Largest mesoperidial crystals of COD on the endoperidial surface 6–13 μm diam, bipyramidal. Peristome sulcate, distinctly delimited or not, with about (17–)22–52(–60) folds. Stalk stout to more or less slender, (2.5–)3.0–9.0(–9.5) mm high and 2.0–8.0(–9.0) mm wide, cream to rather dark brownish grey. Base of the endoperidial body from smooth to plicate or conspicuously sulcate. Basidiospores globose, (5.5–)6.0–7.5(–8.5) μm diam, with 0.6– 1.5(–1.6) μm high warts, ornamentation baculate-pilate. Broadest capillitial hyphae (5.5–)6.0–9.0(–9.5) μm diam. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Macroscopic characteristics — Unexpanded basidiomata up to 30 mm diam, subglobose, with a rounded apex or a flat umbo. Exoperidium splitting in 5–12 more or less equal to unequal rays, 30–107 mm diam apparently, 45–125 mm diam when forced in horizontal position, arched, often more or less hygrometric. Mycelial layer rather thick, whitish to pale cream or somewhat yellowish, strongly intermixed with debris from the substrate, more or less strongly adhered to the fibrous layer but sometimes peeling-off in some parts. Fibrous layer papyraceous to coriaceous when denuded, whitish to cream coloured. Pseudoparenchymatous layer whitish to greyish cream, not or only superficially cracked, < 0.5 mm thick in dry state, about 1 mm thick when fresh, rather persistent. Endoperidial body globose, subglobose, discoid or broadly ellipsoid, rarely irregular, 12–33 mm diam, cream to brownish grey, rarely dark brown; endoperidial surface glabrous. Mesoperidium as a very thick, powdery to creamy, layer of whitish, pale brownish or greyish pruina. Peristome sulcate, with the same colour as the endoperidial surface, narrowly to broadly conical, distinctly or indistinctly delimited. Stalk present, stout to more or less slender, (2.5–)3.0–9.0(–9.5) mm high, cream to rather dark brownish grey. Apophysis present or absent, with the same colour as the endoperidium or slightly darker, smooth to plicate or conspicuously sulcate. Columella intruding beyond the middle into the glebal mass, more or less fusiform, with an acute apex. Mature gleba brownish to dark greyish brown.
Microscopic characteristics — Basidia not seen. Basidiospores globose to subglobose, (5.5–)6.0–7.5(–8.5) μm diam, brownish to yellowish brown, with 0.6–1.5(–1.6) μm high brown warts, ornamentation baculate-pilate. Broadest capillitial hyphae (5.5–)6.0–9.0(–9.5) μm diam, aseptate, very rarely branched, normally straight, thick-walled (2.0–4.0 μm thick), with narrow lumen but mostly visible; tips acute to rounded; surface often covered or not with debris. Endoperidium composed of 2.0–5.5 μm wide, pale yellowish to yellowish brown, aseptate, mostly unbranched, slightly sinuous, strongly intertwined, thick-walled hyphae, lumen visible; protruding hyphae not seen. Peristomal hyphae 2.0–6.0 μm wide, light brown, aseptate, mostly unbranched, thick-walled (1.0–2.5 μm thick), lumen visible or not, often sinuous, narrowing at base and apex, tips mostly acute, a few rounded. Mesoperidium present on the endoperidium and pseudoparenchymatous layer surfaces, consisting of small, bipyramidal crystals of COD, the largest 6–13 μm diam, intermixed with abundant 1.0–3.0 μm wide, hyaline, branched, thin-walled, clamped hyphae. Pseudoparenchymatous layer of thin-walled (≤ 1 μm thick), hyaline to yellowish cells, variable in shape and size, about 10–65 × 8–50 μm diam. Fibrous layer with 1.5–4.5 μm wide, hyaline to pale yellowish, aseptate, straight or slightly sinuous, intertwined, mostly unbranched, comparatively thick-walled (0.7–2.0 μm thick) hyphae, lumen visible. Mycelial layer double-layered; inner layer consisting of 1.0–3.0 μm wide, strongly glued together, more or less hyaline, branched, thin-walled and clamped hyphae; outer layer with 1.0–3.5(–4.5) μm wide, hyaline to pale yellowish, aseptate, rarely branched, comparatively more or less thick-walled (0.5– 2.0 μm thick) hyphae, lumen very narrow and difficult to see. Rhizomorphs with rose-like aggregates of bipyramidal crystals.
Ecology & Distribution — This species grows on both calcareous and siliceous soils, not rarely in anthropized environments, and seems to be characteristic of the ‘Mediterranean forests, woodlands and scrub’ biome of the Paleartic ecozone.
Additional specimens examined (paratypes). PORTUGAL, Estremadura, Mata do Solitario, 12 Nov. 1991, F.D. Calonge, MA-Fungi 31164. – SPAIN, Cáceres, Almaraz, mediterranean woodland, calcareous soil, 17 Jan. 2014, C. Gelpi, herb. Zamora 617; Cádiz, Grazalema, Llanos del Campo, 30STF8170, 760 m, bosque mixto Quercus faginea y Ceratonia siliqua, 15 Dec. 2003, A. Castro 688, MA-Fungi 59644; Canary Islands, Tenerife, Las Mercedes, Cruz del Carmen, 926 m, en bosque de layal-brezal, 30 Dec. 2007, D. Chavez, V. Escobio, J. Caridad & M.Á. Ribes, herb. Ribes 301207-43; Córdoba, Cabra, encinar, 4 Nov. 1990, unknown legit, AH 12674; Córdoba, arroyo de Pedroche, UG4499, bajo Quercus rotundifolia, 24 Dec. 1999, A. Castro 6, MA-Fungi 55189; Guadalajara, embalse de El Vado, under Pinus nigra, Cupressus sp., Quercus pyrenaica, Q. rotundifolia, and Gleditsia triacanthos, calcareous soil, 6 Dec. 2007, M. Bueno & A. Zapata, herb. Zamora 276; ibid., under Pinus sp., Cistus sp., Quercus sp. and Cupressaceae, 12 Dec. 2012, M. Bueno & A. Zapata, herb. Zamora 465; Madrid, Ciudad Universitaria, E.T.S.I. de Montes, under Cedrus libani, 13 Nov. 2004, L. Rubio, herb. Zamora 65; ibid., under Cedrus sp., 20 Aug. 2005, J.C. Campos & J. Daniel-Arranz, herb. Zamora 82; ibid., under Cupressus sp., 22 Nov. 2005, J.C. Zamora, MAFungi 63558 (duplo herb. Zamora 118); ibid., under Cupressus sp., 10 July 2006, J.C. Zamora, herb. Zamora 153; ibid., under Cedrus libani, 30 Oct. 2006, J.C. Zamora, herb. Zamora 185; ibid., under Thuja sp., 23 Nov. 2006, J.C. Zamora, herb. Zamora 192; ibid., under Calocedrus decurrens, 17 Oct. 2007, J.C. Zamora, herb. Zamora 262; ibid., under Cedrus sp., 15 Oct. 2008, J.C. Zamora, herb. Zamora 294; ibid., under Eucalyptus sp. and Cupressus sempervirens, 15 Oct. 2008, J.C. Zamora, herb. Zamora 295; ibid., under Cedrus deodara, 14 Nov. 2008, L. Rubio Casas, AH 42179; ibid., under Chamaecyparis lawsoniana, 20 Nov. 2008, J.C. Zamora, herb. Zamora 315; ibid., under Cupressus sempervirens and Mahonia aquifolium, 11 Nov. 2011, J.C. Zamora, herb. Zamora 587; ibid., under Eucalyptus sp. and Cupressus sempervirens, 11 Nov. 2011, J.C. Zamora, herb. Zamora 588; Madrid, parque Pedriza de Manzanares, pinar, 14 Oct. 1995, F.D. Calonge, MA-Fungi 33803; Madrid, San Agustín de Guadalix, suelo húmico de encinar, 4 Dec. 2004, J.C. Campos, MA-Fungi 60901; Madrid, Villaviciosa de Odón, cerca de la Residencia de Ancianos de la C.A.M., bajo Quercus rotundifolia, Oct. 1998, J.C. Zamora, herb. Zamora 1; Mallorca, Artá, 15 Oct. 1990, C. Constantino, MA-Fungi 27661; Mallorca, Esporles, Port d‘es Canonge, 22 Nov. 1987, L. Siquier 1 V, MA-Fungi 20651; Mallorca, Predio ca na Magdalena Noia, Pollensa, J.L. Siquier, MA-Fungi 20834; Mallorca, Son Forté, Artá, 9 Nov. 1991, J.L. Siquier, MA-Fungi 27662; Palencia, Ampudia, bosque de Cupressus arizonica, 26 Jan. 2008, A. García & M. Sanz, AVM 2856; ibid., AVM 2857; Toledo, Quercus rotundifolia, 4 Dec. 2007, J. De Esteban, herb. Zamora 616; Valladolid, parque de las Contiendas, bajo Cupressus arizonica, 28 Mar. 2011, A. García AVM 2708.
Geastrum papinuttii J.C. Zamora, sp. nov. — MycoBank MB810505; Fig. 7, 13c
Type. ARGENTINA, Santiago del Estero, Guasayán, Prosopis sp., 15 Apr. 2012, J.C. Zamora, L. Papinutti & G. Rolón, holotype MA-Fungi 83764!, isotypes in AH 45204! and UPS!
Etymology. The species is dedicated to Dr. L. Papinutti (†), expert on Argentinian earthstars, who greatly helped the first author during his stay in Argentina and collected all the studied specimens with him.
Diagnosis — Exoperidium 12–24 mm diam in horizontal position, arched, divided in 6–11 non-hygrometric rays. Endoperidial body 3.5–6.5 mm diam, mostly dark brown to blackish. Columella with a more or less rounded apex. Mesoperidium very abundant, more or less powdery. Largest mesoperidial crystals of COD on the endoperidial surface 6.0–12 μm diam, bipyramidal. Peristome sulcate, distinctly delimited or not, with 12–20 folds. Stalk 0.5–1.3 mm high, normally greyish brown to brown. Basidiospores globose, 4.0–5.5 μm diam, with 0.3–0.6 μm high warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae 4.5–6.0 μm diam. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Macroscopic characteristics — Unexpanded basidiomata about 5 mm diam, subglobose and with a rounded apex. Exoperidium splitting in 6–11 more or less equal to unequal rays, 7–21 mm diam apparently, 12–24 mm diam when forced in horizontal position, arched, often more or less hygrometric. Mycelial layer thin, whitish to pale cream, strongly intermixed with debris from the substrate, more or less strongly adhered to the fibrous layer but sometimes peeling-off in some parts. Fibrous layer papyraceous to slightly coriaceous when denuded, whitish to pale-cream coloured. Pseudoparenchymatous layer pale cream to greyish cream, not or only superficially cracked, < 0.5 mm thick in dry state, about 1 mm thick when fresh, rather persistent. Endoperidial body subglobose to ellipsoid, rarely irregular, 3.5–6.5 mm diam, dark brown to blackish; endoperidial surface glabrous or almost so. Mesoperidium as a rather thick, more or less powdery, layer of whitish pruina. Peristome sulcate, with the same colour as the endoperidial surface, mostly narrowly conical, 0.5–1.5 mm high, distinctly or indistinctly delimited. Stalk present but often short, 0.5–1.3 mm high, greyish brown to brown, sometimes light coloured. Apophysis present or absent, with the same colour as the endoperidium. Columella intruding about 1/3–1/2 into the glebal mass, with a more or less rounded apex. Mature gleba brownish.
Microscopic characteristics — Basidia not seen. Basidiospores globose to subglobose, 4.0–5.5 μm diam, brownish to yellowish brown, with 0.3–0.6 μm high brown warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae 4.5–6.0 μm broad, aseptate, very rarely branched, normally straight, thick-walled (2.0–2.5 μm thick), with narrow lumen, mostly visible; tips acute to rounded; surface often covered with debris. Endoperidium composed of 2.5–6.5 μm wide, pale yellowish to yellowish brown, aseptate, mostly unbranched, slightly sinuous, strongly intertwined, thick-walled hyphae, lumen visible; protruding hyphae not seen. Peristomal hyphae 2.5–7.5 μm wide, light brown, aseptate, mostly unbranched, thick-walled (1.0–3.0 μm thick), lumen visible or not, more or less straight to sinuous, narrowing at apex, tips mostly acute, some rounded. Mesoperidium present on the endoperidium and pseudoparenchymatous layer surfaces, consisting of very small, bipyramidal crystals, the largest 6.0–12 diam, intermixed with abundant 1.0–2.5 μm wide, more or less hyaline, branched, thin-walled, clamped hyphae. Pseudoparenchymatous layer of thin-walled (≤ 1 μm thick), hyaline to pale yellowish cells, variable in shape and size, about 12.5–65 × 9–35 μm. Fibrous layer with 1.5–4.0 μm wide, hyaline to very pale yellowish, aseptate, straight or slightly sinuous, intertwined, mostly unbranched, comparatively thick-walled (0.7–1.5 μm thick) hyphae, lumen visible. Mycelial layer double-layered; inner layer consisting of 1.5–3.0 μm wide, strongly glued together, more or less hyaline, branched, thinwalled and clamped hyphae; outer layer with 1.0–3.0 μm wide, hyaline to somewhat yellowish, aseptate, rarely branched, comparatively more or less thick-walled (0.5–1.5 μm thick) hyphae, lumen very narrow and difficult to perceive; myceliar projections indistinct from the outer myceliar layer. Rhizomorphs covered with rose-like aggregates of bipyramidal crystals.
Ecology & Distribution — Only found growing under Prosopis spp. in the ‘Tropical and subtropical grasslands, savannas and shrublands’ biome of the Neotropical ecozone.
Additional specimens examined (paratypes). ARGENTINA, Santiago del Estero, Guasayán, Prosopis sp., 15 Apr. 2012, J.C. Zamora, L. Papinutti & G. Rolón, MA-Fungi 86911; ibid., Prosopis sp., 15 Apr. 2012, J.C. Zamora, L. Papinutti & G. Rolón, MA-Fungi 86912.
Geastrum parvistriatum J.C. Zamora & Calonge, Bol. Soc. Micol. Madrid 31: 139. 2007. — Fig. 7, 13b
Type. SPAIN, Madrid, Ciudad Universitaria, near Instituto de Cardiología de Madrid, under Cupressus arizonica var. bonita, 24 Nov. 2006, J.C. Zamora, holotype MA-Fungi 69583!, isotypes K(M) 147057! and PC 0092573! Numerous paratypes were also cited by Zamora & Calonge (2007).
Synoptic description (based on Zamora & Calonge (2007) and studied specimens) — Exoperidium (13–)19–47 mm diam in horizontal position, arched, divided in 5–12 non-hygrometric rays. Endoperidial body 5–16 mm diam, mostly dark brown to blackish. Columella with a rounded, rarely somewhat acute, rather deciduous apex. Mesoperidium very abundant, powdery. Largest mesoperidial crystals of COD on the endoperidial surface 5.5–12.5 μm diam, bipyramidal. Peristome sulcate, distinctly delimited or not, with 7–23 folds. Stalk 0.5–2.5(–3.0) mm high, mostly brownish to dark brown, rarely pale coloured. Basidiospores globose, (4.5–)5.0–7.0(–7.5) μm diam, with (0.4–)0.5– 1.0(–1.2) μm high warts, ornamentation verrucose to irregularly pilate. Broadest capillitial hyphae (5.0–)5.5–8.0(–8.5) μm diam. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — This species grows in siliceous, calcareous and gypsiferous soils, under both coniferous and broadleaf trees, being known only from the central Iberian Peninsula (Zamora & Calonge 2007, Jeppson 2013). Those records belong to the ‘Mediterranean forests, woodlands and scrub’ biome of the Paleartic ecozone.
Additional specimens examined. SPAIN, Alcalá de Henares, bajo Pinus halepensis, suelo calizo, 15 Nov. 2011, B. Zamora & J.C. Zamora, herb. Zamora 551; Madrid, Ciudad Universitaria, delante del Instituto Anatómico Forense, bajo Cupressus arizonica var. bonita, 10 Mar. 2005, J.C. Zamora, MA-Fungi 68580; ibid., under Cupressus arizonica var. bonita, 11 Nov. 2011, J.C. Zamora, herb. Zamora 578; ibid., under Cupressus arizonica var. bonita, 19 Oct. 2012, J.C. Zamora, C. Galán & L. Zhang, herb. Zamora 539; Madrid, Ciudad Universitaria, al lado del Instituto de Cardiología de Madrid, bajo Cupressus arizonica var. bonita, 10 Mar. 2005, J.C. Zamora, MA-Fungi 68583; ibid., bajo Cupressus arizonica var. bonita, 26 May 2005, P. Gancedo & J.C. Zamora, MA-Fungi 68582; ibid., under Cupressus arizonica var. bonita, 11 Nov. 2011, J.C. Zamora, herb. Zamora 577; Madrid, Getafe, cerro de los Ángeles, en pinar, 23 Feb. 2008, L. Penelas, herb. Zamora 285; Madrid, Villaviciosa de Odón, urbanización Campodón, under Olea europaea, 25 Nov. 2007, B. Zamora, herb. Zamora 272; ibid., under Olea europaea, sandy soil, 12 Nov. 2011, B. Zamora & J.C. Zamora, herb. Zamora 580; Toledo, under Quercus rotundifolia, sandy, siliceous soil, 12 Dec. 2010, J. De Esteban, J.C. Zamora & B. Zamora, herb. Zamora 460.
Geastrum pectinatum Pers., Syn. Meth. Fung.: 132. 1801. — Fig. 12c
Type. Lectotype (designated here, MBT198526): f. 11 of pl. XXXVII in Schmidel (1793). Epitype (designated here, MBT198527) that supports the lectotype cited above: SWEDEN, Uppland, Denmark par., between Bergsbrunna Railway Station and Svedden (near Uppsala), on an abandoned ant-hill in coniferous wood, 5 Sept. 1946, A. Melderis, Lundell & Nannfeldt, Fungi Exiccati Suecici 1442, UPS 437414 (F-149330)! (Duplicates (isoepitypes) of this exiccatum were also distributed in the following herbaria: BMI, C, K, LE, PC, PRM, S, W).
= Geastrum calyculatum Fuckel, Jahrb. Nassauischen Vereins Naturk. 37. 1870, ‘Geaster calyculatus‘.
≡ Geastrum bryantii subsp. calyculatum (Fuckel) G. Winter, Dr. L. Rabenhorst’s Kryptogamen-Flora 1: 911. 1884, ‘Geaster [bryantii subsp.] calyculatus’. — Type: Unknown. In absence of original specimens, f. 3 of t. 5 in Fuckel (1870) can be selected as lectotype.
Synoptic description (based on Sunhede (1989) and studied specimens) — Exoperidium 20–135 mm diam in horizontal position, with (4–)6–10(–11) non-hygrometric rays. Endoperidial body (4.5–)10–25(–36) mm diam, dark brown. Mesoperidium very abundant, powdery. Largest mesoperidial crystals of COD on the endoperidial surface 5–15 μm diam, bipyramidal. Peristome sulcate, distinctly delimited or not, with 11–35 folds. Stalk slender to more or less stout, (1.5–)3.5–13(–15) mm high and (1.6–)2.0–6.0 mm wide, mostly light coloured. Base of the endoperidial body from smooth to plicate or conspicuously sulcate. Basidiospores globose, 5.5–7.5(–8.0) μm diam, with 0.6–1.5 μm high warts, ornamentation baculate-pilate. Broadest Capillitial hyphae (8.0–)9.0–13.0(–15.0) μm diam. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — The vast majority of confirmed records of this species came from ‘Boreal forests/taiga’, ‘Temperate conifer forests’, and ‘Temperate broadleaf and mixed forests’ biomes of the Paleartic ecozone (Sunhede 1989).
Additional specimens examined. BELGIUM, sous Picea, 22 Nov. 1964, V. Demoulin 2889, MA-Fungi 43295. – SPAIN, Burgos, Aranda de Duero, 19 Oct. 1982; F. Lanata, MA-Fungi 5745; Burgos, jardines junto a la Cartuja de Miraflores, bajo Pinus sylvestris, 15 Nov. 2007, F.D. Calonge & F. Esteve-Raventós, MA-Fungi 75533; Cuenca, terreno básico, bajo Pinus nigra y Juniperus thurifera, Oct. 1988, G. Moreno & J.L. Manjón, AH 11479; Gerona, Cerdaña, en humus de conífera, 23 Sept. 2008, E. Vernís, herb. Zamora 292; Huesca, Valle de Hecho, selva de Oza, camino de Espata, bajo Fagus sylvatica, Abies alba y Pinus sylvestris, 11 Oct. 2008, F. Palazón, herb. Zamora 290; Lugo, La Rua, bajo Cupressus arizonica, 12 Oct. 1988, J.M. Ruiz, MA-Fungi 21686; Lugo, Monforte de Lemos, 12 Oct. 1991, A. Fernández, MA-Fungi 28156. – SWEDEN, Gotland, Gammelgarn parish, Uppstaig naturreservat, barrskog (coniferous forest), 27 Sept. 2011, T.N. Kristiansen & P. Marstad, UPS F-560803; Gotland, Gothem parish, Jusarve skog, under Picea abies and Pinus sylvestris, calcareous soil, 30 Sept. 2011, J.C. Zamora, herb. Zamora 613.
Nomenclatural notes — This species could be traced in Persoon (1794) as ‘G. multifidum var. α’, a name not validly published according to Art. 32.1(b), see also Ex. 4 of Art. 9.5.
Palmer (1959) designated a neotype from Persoon’s herbarium (see Sunhede 1989) but, according to Art. 9.19(a) of the Melbourne Code (McNeill et al. 2012), it is to be superseded because original material exists. As previously noted by Sunhede (1989), f. 11–14 of pl. XXXVII in Schmidel (1793), which is the only original material known so far, can be selected as lectotype. This is formally done here. Plate XXXVII is composed by several figures, and f. 11 is the one specifically designated as lectotype because only f. 11 and 12 represent the species currently know as G. pectinatum, and in f. 11 the stalk with the pseudoparenchymatous collar as a ring, typical for all species of the G. pectinatum group, is well-visible. However, since some specimens of G. pectinatum s.str. and G. meridionale are not easily distinguished on the basis of morphological characters (see Discussion), an epitype should also be designated to unambiguously set the taxonomic concept of this taxon, in order to prevent future discussions about its interpretation, and preserve the current usage of the name. We prefer to select a sequenced, not very old specimen, to provide not only morphological but also molecular phylogenetic data, dispelling any uncertainty about its identity. The designated epitype consists of three well-preserved, mature fruitbodies in agreement with Persoon’s (1801) description and with the general concept of this species, excluding the Macaronesian and Mediterranean specimens assignable to G. meridionale. This collection is part of the Fungi Exiccati Suecici, so duplicates are present in different herbaria, easing further revisions. The apophysis of the basidiomata from the epitype varies from smooth to clearly sulcate, so it represents part of the intraspecific variation of the species. In addition, basidiomata were found on an abandoned ant-hill in coniferous wood, which is one of the preferred habitats of G. pectinatum (Sunhede 1989). Geastrum calyculatum is regarded as a synonym based on the chorological, ecological, and morphological data from the protologue (Fuckel 1870), which fit well within the intraspecific variation of G. pectinatum observed during our study.
Geastrum plicatum Berk., Ann. Mag. Nat. Hist. 3: 399. 1839, ‘Geaster plicatus’. — Fig. 12d
Type. Lectotype (selected by Palmer 1959): India, Madras, Dr. Wight (ex herb. Hooker), K(M) 180401!
Synoptic description — Exoperidium 38–62 mm diam in horizontal position, with 6–9 non-hygrometric rays. Endoperidial body 10–18 mm diam, pale to dark brown. Mesoperidium very abundant, more or less powdery. Largest mesoperidial crystals of COD on the endoperidial surface 7–20(–32) μm diam, bipyramidal. Peristome sulcate, distinctly delimited or not, with 16–30 folds. Stalk often very slender, 3.5–6 mm high and 0.7–2.5 mm wide, light or dark coloured. Base of the endoperidial body plicate to strongly sulcate. Basidiospores globose, 5.0–6.5 μm diam, with 0.7–1.2 μm high warts, baculate-pilate ornamentation. Broadest capillitial hyphae 6.0–8.0 μm diam. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — In the broad sense followed in the present study, this species grows in humic soils. The studied specimens come from ‘Tropical and subtropical moist broadleaf forests’, ‘Tropical and subtropical grasslands, savannas and shrublands’, ‘Temperate grasslands, savannas and shrublands’, and ‘Montane grasslands and shrublands’ biomes of the Neartic, Paleartic, and Indo-Malay ecozones (Berkeley 1839).
Additional specimens examined (as G. cf. plicatum, which are included provisionally under G. plicatum until specimens from the Indo-Malay ecozone become available for further studies, see Discussion). ARGENTINA, Buenos Aires, partido Lomas de Zamora, Llavallol, Santa Catalina, Ligustrum lucidum implanted forest, 12 May 2012, F. Kuhar, V. Castiglia, E. Grassi & J.C. Zamora, MA-Fungi 83774; Entre Ríos, Colón, Ubajay, marginal forest, 2 June 2012, J. Maller & J.C. Zamora, MA-Fungi 87322; Salta, Yungas, 11 Apr. 2012, L. Papinutti, J.C. Zamora & G. Rolón, MA-Fungi 87323. – TANZANIA, Iringa, Njombe, c. 4 km W of Ibumila on Njombe-Kidugale road, S09:10 E34:35, 1820 m, low Uapaca-Brachystegia woodland, 3 June 1985, B. Pettersson, M. Hedrén & S.P. Kibuwa 791, UPS F-09935. – URUGUAY, Candones, silv. Cupress, humus aren. sicc. ill. foresta, 40 m, July 1946, Herter, PL. UR. 1908a, Ex. herb. Hert 61024, S.
Geastrum striatum DC., Fl. Franc. (DC. & Lamarck), ed. 3, 2: 267. 1805, ‘Geaster striatus’ — Fig. 13d
Type. Lectotype (designated here, MBT198542): f. 19 in Bryant (1782).
= Geastrum bryantii Berk., The English Flora of Sir James Edward Smith 5, 2: 300. 1836, ‘Geaster bryantii’. — Type: Unknown. In absence of original specimens, f. 19 in Bryant (1782) can be selected as lectotype, in which case the name would become a homotypic synonym of G. striatum DC.
= Geastrum orientale Hazsl., Grevillea 6, 39: 108. 1878, ‘Geaster orientalis’. — Type: Unknown. In absence of original specimens, f. 12–15 of pl. 98 in Hazslinszky (1878) can be selected as lectotype.
= Geastrum bryantii subsp. kunzei G. Winter, Dr. L. Rabenhorst’s Kryptogamen-Flora 1: 911. 1884, ‘Geaster’. — Type: Unknown.
= Geastrum striatum f. rufidum V.J. Staněk in Pilát, Flora ČSR B-1: 461, 787. 1958. — Type: CZECH REPUBLIC, Louny, 1 Nov. 1952, M. Křížová, holotype PRM.
? = Geastrum bryantii var. minor Berk. in Massee, Brit. Fungus-Fl. 1: 37. 1892, ‘Geaster’. — Type: Unknown. Besides, Berkeley (in Massee 1892) did not provide bibliographic references or figures.
Synoptic description (based on Sunhede (1989) and studied specimens) — Exoperidium 21–85 mm diam in horizontal position, arched, with (4–)6–10(–12) non-hygrometric rays. Endoperidial body 5.5–26 mm diam, mostly brown to black. Mesoperidium very abundant, powdery. Largest mesoperidial crystals of COD on the endoperidial surface 5–15 μm diam, bipyramidal. Apophysis strongly developed, solid, ring-like, with a sharp and acute edge. Peristome sulcate, more or less distinctly delimited or not, with 19–36 folds. Stalk (1.0–)2.0–9.0 mm high, whitish to brownish. Basidiospores globose, 5.0–6.0 μm diam, with 0.5– 1.0 μm high warts, ornamentation verrucose to irregularly pilate. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Commonly growing in anthropized environments, in humic soils often under coniferous, but also broadleaf trees. Mainly distributed in ‘Boreal forests/taiga’, ‘Temperate conifer forests’, ‘Temperate broadleaf and mixed forests’, and ‘Mediterranean forests, woodlands and scrub’ biomes of the Neartic and Paleartic ecozones (Sunhede 1989).
Additional specimens examined. SPAIN, Madrid, Ciudad Universitaria, near Instituto de Cardiología de Madrid, under Cupressus arizonica var. bonita, 8 Oct. 2007, J.C. Zamora, herb. Zamora 242; Madrid, Ciudad Universitaria, E.T.S.I. de Montes, bajo Pinus halepensis, 9 Jan. 2007, J.C. Zamora, herb. Zamora 212; ibid., bajo Cupressus sp., Mahonia aquifolium, Pinus sp. y otras plantas, 9 Oct. 2007, J.C. Zamora, herb. Zamora 250; ibid., under Pinus halepensis, 17 Oct. 2007, J.C. Zamora, herb. Zamora 251; ibid., bajo Cupressus sp., 18 Oct. 2012, J.C. Zamora, L. Zhang & C. Galán, herb. Zamora 540; ibid., bajo Pinus halepensis, 18 Oct. 2012, C. Galán, L. Zhang & J.C. Zamora, herb. Zamora 541; Valladolid, Villanueva de Duero, encinar de Quercus rotundifolia, sombrío, húmedo y arenoso, con abundante y gruesa capa de humus, 2 Nov. 2007, G. Martínez & A. García, herb. Zamora 257. – Sweden, Uppland, Uppsala, humus of Abies sp., 23 Oct. 2011, J.C. Zamora, MA-Fungi 86672.
Nomenclatural notes — In agreement with Sunhede (1989), the names G. bryantii and G. orientale are considered syno 159 nyms due to data from the protologues, as well as G. bryantii subsp. kunzei. Geastrum striatum f. rufidum is also reduced to a heterotypic synonym since the presence of reddish tints in the exoperidium is part of the natural intraspecific variation of G. striatum. Geastrum bryantii var. minor is only tentatively treated as a heterotypic synonym because the data of the protologue are vague, so it is not clear which taxon was described due to the existence of an European morphologically close species (G. parvistriatum) that often produces smaller basidiomata. Finally, Sunhede (1989) noted that f. 19 in Bryant (1782) could be selected as lectotype for G. striatum, but he did not finally lectotypify the species, which is formally done here.
Geastrum aff. striatum DC. — Fig. 13e
Synoptic description — Exoperidium 28–29 mm diam in horizontal position, more or less arched, with 8–9 non-hygrometric rays. Endoperidial body 9.0–9.5 mm diam, blackish. Mesoperidium very abundant, powdery. Largest mesoperidial crystals of COD on the endoperidial surface about 12–13 μm diam, bipyramidal. Apophysis well-developed, solid, ring-like, with a rounded edge. Peristome sulcate, more or less distinctly delimited, with 14–24 folds. Stalk 1.6–2.0 mm high, whitish to brownish. Basidiospores globose, 4.5–5.0 μm diam, with 0.4–0.8 μm high warts, ornamentation verrucose to irregularly pilate. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Insufficiently known; the single studied collection was found in the ‘Tropical and subtropical dry broadleaf forests’ biome of the northernmost part of the Neotropic ecozone.
Additional specimens examined. MEXICO, Baja California, sierra La Laguna, bajo Lysiloma candida y Pachycereus pringlei, 8 Nov. 1984, C. Ochoa AH 18521 (duplo BCMEX 1755).
Geastrum tenuipes Berk., London J. Bot. 7: 576. 1848, ‘Geaster’ — Fig. 12e
≡ Geastrum pectinatum var. tenuipes (Berk.) Cleland & Cheel, J. Proc. Roy. Soc. New S. Wales 49: 227. 1915. — Type: Lectotype (selected by Palmer 1959): Aus tralia, Tasmania, Gunn 1778 (ex herb. M.J. Berkeley), K(M) 180399!
Synoptic description — Exoperidium 25–52 mm diam in horizontal position, with 6–11 non-hygrometric rays. Endoperidial body 8–17 mm diam, greyish to dark brownish grey. Mesoperidium very abundant, slightly powdery or not. Largest mesoperidial crystals of COD on the endoperidial surface (20–)25–55 μm diam, bipyramidal. Peristome sulcate, often distinctly delimited, with 14–27 folds. Stalk very slender, 3.0–7.0 mm high and 0.7–1.5 mm wide, light or dark coloured. Base of the endoperidial body smooth to plicate or clearly sulcate. Basidiospores globose, 5.5–7.5(–8.5) μm diam, with 0.7–1.3(–1.5) μm high warts, ornamentation baculate-pilate. Broadest capillitial hyphae 6.0–9.5 μm diam. Pseudoparenchymatous layer of thin-walled (mostly ≤ 1 μm thick) cells.
Ecology & Distribution — Growing on natural and anthropized environments, records come from the ‘Temperate broadleaf andmixed forests’ biome of the Australasian ecozone (Berkeley 1848, Cunningham 1944).
Additional specimens examined. AUSTRALIA, Australian Capital Territory, Canberra, suburb of Macquarie, S35°15' E149°04', 600 m, suburban garden, in dense leaf litter, 7 Nov. 2004, H. Lepp 4603, CANB 745974; Australian Capital Territory, Canberra, Mauldon Street, Chifley, S35°21'20" E149°04'35", 635 m, suburban garden, among leaf and woodchip in garden beds, 5 July 2008, J. Palmer 760, CANB 775658; Australian Capital Territory, Kaleen, Canberra, S35°13' E149°06', in a suburban garden, on ground, May 2006, C. Evans 9, CANB 738350.
DISCUSSION
An unexpected diversity unravelled by molecular and morphological data
In the recent taxonomic circumscription of the genus Geastrum proposed by Zamora et al. (2014), Geastrum sect. Geastrum comprised about 17–18 well-defined phylogenetic lineages, but only 10 species names, i.e., G. arenarium, G. coronatum, G. glaucescens, G. minimum, G. ovalisporum, G. parvistriatum, G. pectinatum, G. quadrifidum, G. smithii, and G. striatum, are more or less broadly known and accepted or used in recent studies (Bates 2004, Fazolino 2009, Jeppson et al. 2013, Kuhar et al. 2013). In the present study 27 well-defined phylogenetic lineages are considered as potential species, which means almost three times the number of the ‘classical species names’ previously mentioned. Despite not all specimens could be unequivocally assigned to a species name, we have been able to name 23 species, seven of them proposed as new (G. austrominimum, G. benitoi, G. britannicum, G. kuharii, G. meridionale, and G. thanatophilum), plus another one (G. leptospermum) recognized by morphological features but not included in molecular analyses, because only the old and scanty material of the type specimen was available. The four subsections already defined by Zamora et al. (2014) are retrieved here and confirmed as well-defined by both morphological and molecular data. Geastrum subsect. Hungarica is newly proposed for accommodating the morphologically remarkable G. hungaricum (see Taxonomy).
As noted in the results, the single specimen TNS TKG-GE-91002, whose molecular data were collected from GenBank, showed a phylogenetic position intermediate between G. subsect. Hungarica and G. subsect. Quadrifida. In the GenBank database this specimen is available under the name ‘G. quadrifidum’, so one may be tempted to directly include it within G. subsect. Quadrifida, a decision that would not be against our phylogenetic reconstructions (Fig. 1). Unfortunately, this specimen was not available to us for morphological study. Therefore, making such taxonomic decision without morphological data is discouraged, because this specimen may show morphological characters so strongly deviating from other species included in G. subsect. Quadrifida, for deserving a new subsection. In fact, as an example, G. britannicum sequences were also available as G. quadrifidum in GenBank, but the species is best included in G. subsect. Arenaria (Fig. 1). For these reasons, the specimen TNS TKG-GE-91002 is left taxonomically unplaced.
Due to the complexity of the entire sect. Geastrum, as a result of the number of taxa involved and the numerous taxonomic and nomenclatural problems found during this study, comments and possible solutions proposed for each subsection and species are separately addressed below.
Subsect. Arenaria
This subsection is mainly characterized by the shape and size of the basidiospores, as well as by the often less marked ornamentation in comparison with the remaining ones (Zamora et al. 2014). Five species are included within this group in the present treatment, of which G. leptospermum is included by morphological data only.
Geastrum ovalisporum, described by Calonge et al. (2000), is probably the most clearly differentiated species, being the ovoid, small and inconspicuously ornamented basidiospores the most distinctive morphological character (Fig. 9e5). The studied specimens are morphologically rather homogeneous; the dark endoperidial body and the whitish, often longer than broad stalk seem to be reliable characteristics as well.
The South European specimens of the so-called G. arenarium form a clear and well-supported clade according to our phylogenetic analyses (Fig. 1), and deviate from the American taxon which we regarded as G. arenarium s.str. The mesoperidial crystals of these South European samples are often in the form of bipyramidal prisms with very conspicuous faces even under the light microscope, mixed with some bipyramids, while in the American samples such prisms with conspicuous faces have not been seen. The disparate distributional and ecological ranges contribute to support the independence of two species. Then, the South European samples are separated as a new species, which is described in the taxonomic part as G. benitoi. Although the morphological comparisons of basidiospore size and ornamentation height also revealed significant differences, such differences are of the order of tenths of micrometers and, especially in the case of the ornamentation height, a practical separation is not easy. Therefore, it can be only said that basidiospores in G. arenarium tend to be somewhat smaller and less ornamented than in G. benitoi. Fortunately, and as noted in the results, G. benitoi is additionally distinguished because of its pseudoparenchymatous cells with thin walls, while the American G. arenarium specimens have more or less thick walls. According to Sunhede (1989), the thickness of the pseudoparenchymatous cell wall is related to the hygrometric behaviour of the exoperidial rays, and therefore only G. arenarium can be considered as truly hygrometric. In fact, G. arenarium s.str. is the only species in G. sect. Geastrum, together with G. hungaricum, that has a truly hygrometric exoperidium. Nevertheless, G. hungaricum is clearly distinguished by its much bigger basidiospores, pseudoparenchymatous cells with thicker walls, more strongly hygrometric exoperidium, mycelial layer peeling off very easily, and different crystalline matter of the mesoperidium, which is composed almost exclusively by crystalline aggregates of COM (Fig. 9f3). It should be noted that the South American specimens of G. arenarium often tend to have pseudoparenchymatous cells with slightly thicker walls in comparison with the North American specimens, while the basidiospores are almost identical. Since few herbarium specimens have been studied, we do not know if such subtle differences are consistent, and thus we prefer to treat both groups as a single species. All in all, it will not be surprising that future studies allow the distinction of additional species in this complex.
Geastrum britannicum is superficially close to G. quadrifidum (G. subsect. Quadrifida) by macromorphological traits, but is better included within G. subsect. Arenaria due to both molecular data and basidiospore features. In fact, there are several morphological characteristics that can be used to separate G. britannicum from G. quadrifidum. In G. britannicum the largest crystals that cover the endoperidial surface are comparatively big (63–80 μm diam) and they are usually grouped and fused forming rounded, 60–120 μm diam aggregates, thus giving a grainy appearance to the endoperidial body, while in G. quadrifidum the endoperidium is covered by smaller ((10–)15–50(–60) μm), not aggregated, bipyramidal crystals, which confer a pruinose appearance to the endoperidial body. Morphological analyses also identified that basidiomata size tends to be slightly larger in G. britannicum, but too few basidiomata were included in the analyses to consider this a reliable character. The same applies to the stalk length, which seems to be slightly longer in G. britannicum. Another macromorphological trait to distinguish both species is the often inconspicuously folded peristome in G. britannicum, which is purely fibrillose in G. quadrifidum. In addition, G. quadrifidum has globose, (4.5–)5.0–6.0 μm diam basidiospores, with very conspicuous, up to 0.8 μm high warts. In contrast, G. britannicum has globose to slightly ovoid, (3.5–)4.0–4.5(–5.0) μm diam basidiospores, with less conspicuous, up to 0.5(–0.6) μm high warts.
Geastrum leptospermum is one of the smallest earthstars, with a distinctive combination of morphological characteristics. Together with G. britannicum and G. quadrifidum, this is the only other fornicate species in G. sect. Geastrum, with basidiospores somewhat resembling those of G. britannicum, although smaller and less ornamented. It also differs by the much smaller basidiomata, very short stalks, absence of big mesoperidial crystal aggregates, and lighter coloured glebal mass (Atkinson 1903, Coker & Couch 1928, Sunhede 1989). The striking ecology is very different as well, because G. leptospermum grows on mossy bark of living trees instead of growing on soil (Atkinson 1903, Coker & Couch 1928). Interestingly, we have observed many prism-shaped crystals probably of COM on the rhizomorph surface, forming stellate aggregates (Fig. 2f), in addition to some bipyramidal crystals of COD. Such crystals were previously known only in species of G. sect. Myceliostroma, in which they do not form stellate aggregates but are covering cystidioid cells (Zamora et al. 2013). The presence of crystal morphotypes assignable to COM in G. sect. Geastrum is not uncommon, since horn-like crystals have been recorded for G. ovalisporum, G. subsect. Sulcostomata, and G. coronatum, being the main type in the last species (Zamora et al. 2013).
Subsect. Geastrum
The subsect. Geastrum, which includes the type of the genus, G. coronatum, groups rather stout species with complex basidiospore ornamentation, unique in the genus, made up by big processes covered by minute rounded warts (Zamora et al. 2014), although transitions with subsect. Quadrifida may be observed. The mesoperidium is not powdery, and mesoperidial generative hyphae tend to dominate over crystals, which are sometimes absent.
Geastrum smithii is readily distinguished by its regularly sulcate and sharply delimited, flat peristome. However, specimens sharing this morphology and included in the present study are not completely homogeneous, and probably they represent more than one taxon. While the Neartic and Paleartic specimens are morphologically very close, the Australasian specimen differs mainly by the slightly less stout stalk and the bigger basidiospores (Fig. 10d3, d5). In addition, mesoperidial crystals are particularly big in the Australasian specimen, but too few basidiomata have been studied to draw conclusions. The phylogenetic analyses also show several differences in sequence data (Fig. 1). For these reasons we cannot discard that this specimen may belong to a close, but actually different, perhaps undescribed species. Further specimens and molecular data are needed to solve the taxonomy of G. smithii s.l.
On the other hand, the G. coronatum general morphology is shared with G. thanatophilum. The true G. coronatum is a rather common species in Europe, which can be distinguished by the robust and mostly medium-sized basidiomata, the fibrillose peristome, dark stalk, and glabrous endoperidial surface, covered with a ‘mealy’ mesoperidium formed by abundant but rather indistinct generative hyphae (Fig. 10a; Sunhede 1989). The rhizomorph crystals are also rather unusual; they are horn-like formations of COM often grouped into arachnoid aggregates (Fig. 2e; Zamora et al. 2013).
Finally, G. thanatophilum is readily distinguished from G. coronatum on account of rhizomorph crystals, mesoperidial features, and capillitial diameter, although old basidiomata of both species may be easily confused. Then, peristomal characteristics may be of some utility when G. thanatophilum has a finely sulcate peristome, because in G. coronatum it is always fibrillose. In addition, G. thanatophilum is, on average, a rather smaller species (Fig. 4d, e, f). Geastrum kuharii is another morphologically close species, which belongs to G. subsect. Quadrifida, and it is distinguished by the smaller basidiospores, with a less complex ornamentation, as well as by the common presence of crystalline aggregates of COM in the mesoperidium (Fig. 2c and see below).
Subsect. Hungarica
This subsection is currently formed by a single species, G. hungaricum. This species shares all of the basic traits of G. sect. Geastrum as firstly noted by Zamora et al. (2014), except the presence of stalk, and is clearly clustered within this section in the present phylogenetic analyses (Fig. 1), but cannot be properly included in any of the previously defined subsections. Its phylogenetic position is close to G. subsect. Quadrifida, but it posses a remarkable morphology, with a sessile endoperidial body, a strongly hygrometric exoperidium and clearly thickwalled pseudoparenchymatous cells, rather big basidiospores, and mesoperidial crystalline matter formed mainly by crystalline aggregates of COM. This combination of morphological characters led us to separate it in a new subsection proposed here as G. subsect. Hungarica. In relation with the whole genus, G. hungaricum can be seen as a morphologically isolated species that has been considered related to G. floriforme by Staněk (1958). However, both species clearly differ from each other, e.g., on the surface of the endoperidium, which is an important feature for distinguishing several taxa (Sunhede 1989) and for the infrageneric classification of the genus Geastrum (Zamora et al. 2014). For that reason, it would have been difficult to include G. hungaricum in G. sect. Papillata, whose type is G. floriforme. Geastrum corollinum is another morphologically close species but the mycelial layer does not encrust debris and the basidiospore ornamentation is rather different (Sunhede 1989). In fact, species in G. sect. Corollina as defined by Zamora et al. (2014), whose type is G. corollinum, mostly show a rather regular pilate-baculate ornamentation, in contrast to the irregularly verrucose ornamentation of G. hungaricum (see Fig. 9f4 and Sunhede 1989: 232), which is much closer to that of members of sect. Geastrum, and particularly of G. minimum s.l. (see Fig. 11a5, b5, c5, c5, d5, e5 and Sunhede 1989: 265). The crystalline aggregates of COM present on the endoperidial surface are another important morphological character that places G. hungaricum closer to G. subsect. Quadrifida than to G. sect. Papillata or G. sect. Corollina.
Subsect. Quadrifida
This subsection is a morphologically rather homogeneous group clustering all the remaining species with a fibrillose peristome. It is considerably similar to G. subsect. Arenaria, mainly differing in basidiospore shape, size, and ornamentation (Zamora et al. 2014). In addition, crystalline aggregates of COM are often present in some species of this section, but have never been observed in G. subsect. Arenaria.
Geastrum kuharii has a remarkable morphology, rather different to other taxa included in G. subsect. Quadrifida, due to the large and stout basidiomata (Fig. 10e1, e2, e3), which strongly remind of species in G. subsect. Geastrum, particularly G. thanatophilum as has been commented before. However, basidiospore ornamentation is typical of G. subsect. Quadrifida, not as complex as in G. subsect. Geastrum, and basidiospores are also smaller, even on the borderline with G. subsect. Arenaria (Fig. 10e5). Furthermore, crystalline aggregates of COM are often present on the endoperidial surface (Fig. 2c) while, as noted above, they have never been seen in species of G. subsect. Geastrum and G. subsect. Arenaria.
Geastrum quadrifidum is a well-known and well-defined species. The fornicate exoperidium, with mostly 4–5 rays of conspicuously rolled margins (Fig. 11f1), is a reliable characteristic (Sunhede 1989). The other fornicate species of G. sect. Geastrum, i.e., G. leptospermum and G. britannicum, have smaller and less ornamented basidiospores, and bigger mesoperidial crystals (Fig. 3d, e, f), as previously discussed.
While G. kuharii and G. quadrifidum are clearly defined species, the remaining taxa of this group have been considered conspecific and treated under the collective name ‘G. minimum’ by many authors. In the present treatment, this ‘G. minimum group’ of putatively cryptic species includes G. austrominimum, G. calceum s.l., G. granulosum, and G. marginatum. Thus, considering that the name G. minimum has been largely applied to several species, that the protologue is not enough detailed to know which species was described by Schweinitz (1822), and that it is difficult to ascertain if the old specimen marked as ‘type’ in Kew is really a part of Schweinitz’s original material (Sunhede 1989), we prefer to avoid the use of that name, regarding it as nomen ambiguum and dubium until unambiguous original material of this taxon were found. For these reasons, comments on the taxonomy of this group are needed to make a proper distinction of the different taxa. Nevertheless, these four taxa can be distinguished rather well through morphometric analyses (Fig. 5, Table 2), so they are better referred to as pseudo-cryptic species (Mann & Evans 2007, Medina et al. 2012). Nomenclatural notes on certain species are provided in the taxonomic part.
To begin with, all the studied Australasian specimens of ‘G. minimum s.l.’ are grouped in a well-defined clade (Fig. 1). Although somewhat variable and close to G. marginatum and G. granulosum, those specimens are morphologically distinguished by the combination of more or less dark coloured stalks, the often rather big bipyramidal crystals of COD, and particularly by the big, yellowish crystalline aggregates of COM on the endoperidial surface (Fig. 6e, 11a4). Morphometric analyses show that G. marginatum can be distinguished mainly by the smaller crystals of COD and crystalline aggregates of COM on the endoperidial surface (Fig. 6d, e). In G. granulosum the yellowish crystalline aggregates of COM are almost absent or are much smaller, although exceptions can be found (Fig. 6e). Fortunately, G. granulosum basidiomata show, in addition, light coloured stalks as a distinctive qualitative character (Fig. 11d3). Rarely, basidiomata of Australasian specimens may show dominant and very big mesoperidial crystals of COD, and then the DFA identified these fruitbodies as belonging to G. granulosum (Fig. 5a). These basidiomata belong to the voucher MEL 2292062, which is one of the specimens included in the molecular analyses, nesting with the remaining Australasian specimens, and not with G. granulosum (Fig. 1). As a result, the Australasian specimens are described here as the new G. austrominimum.
Lloyd (1907) described G. calceum (as Geaster calceus) based on specimens from South Africa. The description is not very detailed, but he considered the species as close to G. minimum but being larger and with the endoperidial surface densely covered by white granules. Luckily, the iconography (pl. 95) shows one basidioma with a well-developed, more or less dark-coloured stalk, a well-formed apophysis, and a very dense crystal covering on the endoperidial surface. This basidioma strongly reminds the specimens included in this study as G. calceum s.l. However, we have found an unexpected molecular variation between the two sequenced specimens, which may indicate that they are different species (Fig. 1). From a morphological point of view, the specimen MA-Fungi 83761 (G. cf. calceum1) has slightly bigger basidiospores with larger warts and slightly stouter stalks than UFRN-Fungos 723 and MA-Fungi 65435 (G. cf. calceum2) (Fig. 11b3, b4, c1, c2, c3, b5–c5). The ecology and distribution seem to be different, because G. cf. calceum1 was found in the ‘Tropical and subtropical grasslands, savannas and shrublands’ biome, while both specimens of G. cf. calceum2 came from the more humid ‘Tropical and subtropical moist broadleaf forests’ biome. Unfortunately, only three collections of G. calceum s.l. were available for morphological study, and among them there was no South African material. Therefore, further data are needed before conclusions on the taxonomy of this species can be drawn. These specimens can be separated from other species of the G. minimum group mainly by the well-marked apophysis (Fig. 11b3, b4, c1, c2, c3), which is almost ring-like, but also by the ornamentation height and the average basidiomata size (Fig. 6b, c, f). The comparatively slender and long stalks are of some utility, too. Despite the five basidiomata included in DFA are classified by such analysis as distinct from the remaining species of the G. minimum group, it is the only species for which Cohen’s kappa test (Titus et al. 1984) was not significant (Table 2), undoubtedly due to the small sample size. Once more, this data indicate that more specimens of this taxon should be studied.
Fuckel (1860) described G. granulosum (as Geaster granulosus) from specimens collected in Germany. According to his description, it is a species with fibrillose peristome, and dark, stalked endoperidial body, covered by white granules. Even if the colour of the stalk is not indicated, the white granules, which the epithet ‘granulosum’ refers to, allow the distinction of this species from G. marginatum, the only other species of G. minimum s.l. present in Europe. As indicated in the results, most basidiomata of G. granulosum were correctly identified by DFA. Basidiomata incorrectly assigned to G. marginatum, due to particularly small COD crystals, belong to the voucher MICH 72010, and one of the basidiomata incorrectly classified as G. austrominimum, due to particularly big COM crystalline aggregates, to the voucher Sunhede 7746. Both specimens were sequenced and they are placed in the same clade as the remaining G. granulosum specimens (Fig. 1).
Geastrum marginatum is the last species of the G. minimum group. Basidiomata typically possess dark stalks (except some very small basidiomata), and in morphometric analyses it is distinguished from G. austrominimum and G. granulosum mainly by the sparser and smaller mesoperidial COD crystals and COM crystalline aggregates. Geastrum austrominimum has a very different distribution as commented before, and G. granulosum basidiomata showed light coloured stalks. The few basidiomata incorrectly classified by DFA due to particularly big crystalline elements on the endoperidial surface, showed clearly dark stalks, which prevents confusion with G. granulosum. When describing the species, Vittadini (1842) included the following locotypic indication: “In locis aridis arenosis una cum Geastre Schmideli circa Papiam frequens. Autumno”. Geastrum marginatum as conceived in the present study often grows on sandy, siliceous, lime-free to slightly calcareous soils, while G. granulosum seems to be restricted to, or at least clearly prefers, markedly calcareous soils.
Subsect. Sulcostomata
This subsection is well-characterized by the sulcate peristome and the very well-developed mesoperidium, as a powdery layer of very small crystals and generative hyphae on both the endoperidial and pseudoparenchymatous layer surfaces (Zamora et al. 2014). This group is surprisingly diverse and, although only G. pectinatum and G. striatum are normally taken into consideration (plus G. parvistriatum in the most recent treatments), at least 10 taxa can be distinguished according to the results of the present study.
Geastrum striatum is a well-defined species, and the most striking character is the very well-developed, solid, ring-like apophysis, unique in the genus (Fig. 13d3; Sunhede 1989). However, two GenBank sequences not generated by us and clustering within our clade of G. striatum with strong support (Fig. 1) were available at the GenBank database under the name G. pectinatum, which never has a solid, ring-like apophysis. That advises on taking GenBank species names with caution for determination purposes, as many authors haveindicated before for different taxonomic groups (e.g., Nilsson et al. 2006, Shen et al. 2013, Sandoval-Sierra et al. 2014). In addition, during our study we have revised one herbarium collection from Baja California, Mexico (AH 18521) that was determined as G. striatum, and previously illustrated by Ochoa & Moreno (2006). It consists of two basidiomata that deviate from our concept of G. striatum by having less marked apophyses, with rounded edges, and shorter stalks. Basidiospores are also slightly smaller (4.5–5.0 μm diam). This well-separated sample is sister to the clade formed by the G. striatum s.str. samples, and it very likely represents a different species. Nonetheless, having studied only two basidiomata from a single collection, we postpone the formal description of this new taxon until more specimens are available.
Geastrum glaucescens and G. parvistriatum are very close taxa, and their separation is often highly problematic solely based on morphology. While well-defined phylogenetically (Fig. 1), the only reliable morphological characteristic found during our study is the often rounded columella with a deciduous apex in G. parvistriatum (Fig. 13b2), and the normally acuteor pointed columella with a rather persistent apex in G. glaucescens (Fig. 13a2). However, a few basidiomata of G. glaucescens (particularly the smallest ones) have a more or less rounded columella, being almost indistinguishable from most G. parvistriatum basidiomata. In addition, the morphometric analyses showed that the capillitial hyphae tend to be slightly broader and the peristome tends to have more folds in G. glaucescens (Fig. 7d, f), but a wide overlap exists, those differences are not significant, so many specimens cannot be correctly classified by DFA (Fig. 5b). As noted in the results, the only quantitative character that showed significant differences was the basidiospore diameter, likely because of the very high number of measurements taken, but is obviously useless for taxonomic purposes due to the complete overlap (Fig. 7a). By contrast, their disjunct distribution is highly useful to separate them, being G. glaucescens a South American and, up to now Argentinian species, while G. parvistriatum is a South European, to date Spanish species. Argentinian records of G. parvistriatum (Kuhar et al. 2013) should be referred to as G. glaucescens. Taking all these data into account, these two species can be considered semi-cryptic (Vondrák et al. 2009), due to slight differences in morphology and clear dissimilarities in ecology and distribution, but not strictly cryptic in the senses of Sáez & Lozano (2005) and Mann & Evans (2007).
Geastrum papinuttii also belongs to the G. glaucescens-G. parvistriatum group, but it can be distinguished from both of them thanks to the smaller, less ornamented, and paler basidiospores, the thinner capillitial hyphae, and the smaller and more delicate basidiomata (Fig. 7a–e). It is likely that some records of G. glaucescens mentioned by Kuhar et al. (2013) belong to this new species.
Since Palmer’s (1959) revision of the G. plicatum and G. tenuipes type specimens, who considered them as synonyms of G. pectinatum, this last taxon has been treated as a single and well-defined species by morphology in all recent studies (e.g., Sunhede 1989, Calonge 1998, Soto & Wright 2000, Bates 2004, Sarasini 2005, Jeppson et al. 2013). Interestingly, we found a high phylogenetic variation among specimens, strongly related to different geographical origins. This suggests a well-defined geographical structure of several taxa. Therefore, according to our molecular data, it might be possible to distinguish up to five species in this complex, corresponding to four clades from samples sequenced during our study, plus another only recovered using GenBank sequences (Fig. 1). This last clade groups exclusively specimens from the Japanese Paleartic ecozone. It may correspond to G. biplicatum, described from the Bonin Islands by Berkeley & Curtis (1860). Unfortunately, only the single basidioma of the lectotype was available for morphological study. Capillitial hyphae are rather wide, up to 10.5 μm wide, thus approaching to the often very broad hyphae of G. pectinatum (see below). Basidiospores are comparatively small, (4.5–)5.0–6.0 μm diam (Fig. 12a5), thus in the range of G. plicatum, but with a less marked ornamentation than any other species of the G. pectinatum group, with warts hardly reaching 1 μm high. Mesoperidial crystals of COD are small (up to 15 μm diam; Fig. 12a4) as in the other species of G. subsect. Sulcostomata except G. tenuipes and sometimes G. plicatum. Regrettably, the sequenced specimens that might correspond to G. biplicatum due to geographical origin were not available to us for morphological study, and thus their identity is only suspected and could not be confirmed. What is more, while the sequenced specimens belong to the Paleartic ecozone, the type specimen was collected in the Oceanian ecozone, so the existence of more than one species would not be surprising.
The other four phylogenetic clades have been morphologically studied with more detail, and morphometric analyses (Fig. 5, Table 2) indicate a complex of morphologically close, but distinguishable, pseudo-cryptic species (Mann & Evans 2007, Medina et al. 2012). The clade formed by both Paleo- and Neotropical specimens of G. pectinatum s.l. may correspond to G. plicatum. This taxon was described from India, Madras (Berkeley 1839), and referred to as specimens having long and thin stalks and strongly sulcate apophysis or endoperidial basis, in agreement with most of the tropical specimens studied. The basidiospores are one of the smallest of the complex (Fig. 8a, 12d5), while the grooves on the apophysis or the base of the endoperidial body are the most conspicuously marked ones. The type specimen of G. plicatum agrees rather well with the specimens included in our molecular analyses, although the mesoperidial crystals tend to be larger and basidiospores measure 5.5–6.5 μm diam, being transitional with G. tenuipes (see below). This specimen was included in the DFA of the G. pectinatum group (Fig. 5c), clustering with other basidiomata determined as G. plicatum. However, since no samples from the Indo-Malay ecozone have been sequenced, the name G. plicatum is only tentatively assigned to the Neotropical and Afrotropical (Ethiopian) specimens.
The Australasian specimens of G. pectinatum s.l. are phylogenetically well-defined (Fig. 1) and would correspond to the socalled G. tenuipes. This species was described from Tasmania and Australia (Berkeley 1848) based on slender specimens with rather long and thin stalks, and slightly sulcate endoperidial bases. These characteristics match fairly well with the recently collected Australasian specimens revised during this study, although the depth of the basal endoperidial grooves seems variable even within the same collection. The mesoperidial crystals are constantly bigger than in the remaining G. pectinatum group samples (Fig. 8c, 12e4), and seem to be a reliable characteristic for distinguishing this taxon, although they have not been mentioned previously in the literature. Basidiomata from the type specimen are very old and the mesoperidium is almost lost; as a result, the mesoperidial crystal size cannot be evaluated. Basidiospores are, on average, somewhat small, overlapping with G. plicatum, but within the range observed in recently collected specimens of G. tenuipes.
As previously noted, the European specimens of G. pectinatum s.l. form two well-defined clades. One of them belongs to G. pectinatum s.str., a morphologically very variable taxon with mostly slender but sometimes more or less stout stalks, showing also a broad variation concerning the folds of the apophysis (Sunhede 1989). Capillitium hyphae are the widest of the complex (Fig. 8b) and seem to be a reliable characteristic. It is widely distributed through Europe, from the Temperate to the Boreal zones, being common in Central and North Europe. Because Sunhede’s (1989) description was based on a large number of only North European specimens, it is an excellent reference to the true identity of this species. Surprisingly, two specimens from Japan cluster within the G. pectinatum s.str. clade. More data are needed to explain such distribution, but a secondary introduction of the species in that country should not be discarded, as Geastrum specimens are not rarely found in parks and gardens.
The other European clade groups the Macaronesian and Mediterranean specimens, which are close to G. pectinatum but clearly separated by molecular data (Fig. 1). These specimens are described in this study as G. meridionale. Although the representation of the first two discriminant functions of the DFA may give the false impression that these two taxa are hardly distinguished by the studied morphological characters (Fig. 5c), the high percentage of correctly classified samples (95 % of G. meridionale and 100 % of G. pectinatum) indicates that both species are more or less well-separated by morphology. The overlap in the score plot is explained because the third discriminant function is the one that best distinguishes G. pectinatum and G. meridionale. One of the misclassified basidiomata of G. meridionale belongs to the voucher MA-Fungi 20651, which was included in the phylogenetic analyses and clusters together with the remaining samples of G. meridionale (Fig. 1). While G. pectinatum has the thickest capillitium hyphae, G. meridionale has the stoutest stalks of the entire complex (Fig. 8e, 12b3). In addition, the number of peristome folds of G. meridionale is normally much higher, and basidiomata tend to be lighter, especially the endoperidial body, which is mostly cream coloured to brownish, and never blackish as is commonly seen in G. pectinatum. Nevertheless, dark basidiomata of G. meridionale with slender stalks and a low number of peristome folds can be very difficult to distinguish from G. pectinatum, especially when old; then, molecular data become a useful tool for a more precise classification.
To end, since some extensive areas were not sampled (e.g., North America, continental Asia, and Indonesia), other taxa belonging to the G. pectinatum complex might be missing. Further worldwide-based sampling would be needed to propose a more definite taxonomic treatment.
CONCLUSIONS
The example of fungal species delimitation showed with G. sect. Geastrum indicates that, even in hardly morphologically distinguished species, an integrative taxonomy, as a sum of all the taxonomic information sources available (i.a., molecular, morphological, chorological, and ecological data) may result in taxonomic partitions recognizable at species level.
More specifically, in our study, phylogenetic analyses have been basic to establish a solid and objective taxonomic backbone, and to identify nearly cryptic taxa. Discriminant function analyses have been successfully used as a tool for assessing morphological boundaries, and identifying morphological characters that may be useful for species characterization. Ecological and chorological data contribute to support the taxonomic partitions observed.
Acknowledgments
We thank the curators of the studied herbaria for sending specimens on loan, especially to M. Dueñas (MA-Fungi), and all people who contributed directly providing specimens for our studies. Particularly warm thanks are due to people who greatly helped the first author during his stays in Sweden (S. Sunhede, S. Ekman, S. Rymman, M. Myrdal, K. Hansen, and I. Olariaga) and Argentina (L. Papinutti (†), F. Kuhar, V. Castiglia, G. Rolón, E. Grassi, S. Suaza, A. Romero, A. Arambarri (†), and E. García). We thank Yolanda Ruiz for her technical assistance with the SEM. Isabel M. Liberal, P. Jiménez-Mejías and J.V. Sandoval-Sierra kindly provided information on some morphological analyses. Luis A. Parra kindly helped with comments on some nomenclatural issues. Joaquina M. García-Martín is acknowledged for revising the English wording. The first author was supported by a predoctoral fellowship from the Consejo Superior de Investigaciones Científicas (Jae-Pre 2010).
REFERENCES
- Atkinson GF. 1903. A new species of Geaster. Botanical Gazette 36:303–306. [Google Scholar]
- Bartlett MS. 1937. Properties of sufficiency and statistical tests. Proceedings of the Royal Statistical Society, Series A 160: 268–282. [Google Scholar]
- Bates ST. 2004. Arizona members of the Geastraceae and Lycoperdaceae (Basidiomycota, Fungi). Master thesis, Arizona State University, USA. [Google Scholar]
- Berkeley MJ. 1839. Descriptions of exotic fungi in the collection of Sir W J. Hooker, from memoirs and notes of J.F. Klotzsch, with additions and corrections. Annals and Magazine of Natural History, series 1, 3: 375–401. [Google Scholar]
- Berkeley MJ. 1848. Decades of fungi. Hooker's London Journal of Botany 7: 572–580. [Google Scholar]
- Berkeley MJ, Curtis MA. 1860. Characters of new fungi, collected in the North Pacific Exploring Expedition by Charles Wright. Proceedings of the American Academy of Arts and Sciences 4: 110–130. [Google Scholar]
- Borchsenius F. 2007. FastGap 1.0.8. Software distributed by the author. Bottomley AM. 1948. Gasteromycetes of South Africa. Bothalia 4: 473–810. [Google Scholar]
- Brown JM, Hedtke SM, Lemmon AR, et al. 2010. When trees grow too long: Investigating the causes of highly inaccurate Bayesian branch-length estimates. Systematic Biology 59: 145–161. [DOI] [PubMed] [Google Scholar]
- Bryant C. 1782. An historical account of two species of Lycoperdon. London. [Google Scholar]
- Calonge FD. 1998. Gasteromycetes. I. Lycoperdales, Nidulariales, Phallales, Sclerodermatales, Tulostomales. Flora Mycologica Iberica 3: 1–271. [Google Scholar]
- Calonge FD, Moreno-Arroyo B, Gómez J. 2000. Aportación al conocimiento de los Gasteromycetes, Basidiomycotina, de Bolivia (América del Sur). Geastrum ovalisporum sp. nov. Boletín de la Sociedad Micològica de Madrid 25: 271–276. [Google Scholar]
- Calonge FD, Zamora JC. 2003. Geastrum arenarium, encontrado en España y nuevo para Europa. Boletín de la Sociedad Micològica de Madrid 27: 59–61. [Google Scholar]
- Carstens BC, Pelletier TA, Reid NM, et al. 2013. How to fail at species delimitation. Molecular Ecology 22, 17: 4369–4383. [DOI] [PubMed] [Google Scholar]
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17, 4: 540–552. [DOI] [PubMed] [Google Scholar]
- Chajewski M. 2009. rela: Item Analysis Package with Standard Errors. R package version 4.1. [Google Scholar]
- Coker WC, Couch JN. 1928. The Gasteromycetes of the Eastern United States and Canada. The University of Carolina Press, USA. [Google Scholar]
- Cortez VG, Sulzbacher MA, Baseia IG, et al. 2008. Two little known gasteroid fungi from Santa Catarina State, southern Brazil. Mycotaxon 106: 297–302. [Google Scholar]
- Cubeta MA, Echandi E, Abernethy T, et al. 1991. Characterization of anastomosis groups of binucleate Rhizoctonia species using restriction analysis of an amplified ribosomal RNA gene. Phytopathology 81: 1395–1400. [Google Scholar]
- Cunningham GH. 1944. [reprint 1979]. The Gasteromycetes of Australia and New Zealand. Bibliotheca Mycologica 67: 1–239. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, et al. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9, 8: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayrat B. 2005. Towards integrative taxonomy. Biological Journal of the Linnean Society 85: 407–415. [Google Scholar]
- Demoulin V. 1984. Typification of Geastrum Pers. : Pers. and its orthographic variant Geaster (Gasteromycetes). Taxon 33, 3: 498–501. [Google Scholar]
- Edwards DL, Knowles LL. 2014. Species detection and individual assignment in species delimitation: can integrative data increase efficacy? Proceedings of the Royal Society B 281: 20132765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman S, Blaalid R. 2011. The devil in the details: interactions between the branch-length prior and likelihood model affect node support and branch lengths in the phylogeny of Psoraceae. Systematic Biology 60, 4: 541–561. [DOI] [PubMed] [Google Scholar]
- Eyndhoven GL. 1937. Geastrologische notizen. Mededelingen van de Nederlandse Mycologische Vereeniging 24: 12–19. [Google Scholar]
- Fazolino E. 2009. O gênero Geastrum Pers. (Phallomycetidae, Basidiomycota) em algumas áreas de Mata Atlantica e Caatinga no Rio Grande do Norte, Brasil. Master thesis. Universidade Federal do Rio Grande do Norte, Natal, Brazil. [Google Scholar]
- Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- Fisher RA. 1936. The use of multiple measurements in taxonomic problems. Annals of Eugenics 7: 179–188. [Google Scholar]
- Frey-Wyssling A. 1981. Crystallography of the two hydrates of crystalline calcium oxalate in plants. American Journal of Botany 68, 1: 130–141. [Google Scholar]
- Fuckel L. 1860. Enumeratio Fungorum Nassoviae. Jahrbücher des Nassauischen Vereins für Naturkunde 15: 1–123. [Google Scholar]
- Fuckel L. 1870. Symbolae Mycologicae. Jahrbücher des Nassauischen Vereins für Naturkunde 23: 1–459. [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes. Application to the identification of mycorrhizae and rusts. Molecular Ecology 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41: 95–98. [Google Scholar]
- Hazslinszky FA. 1878. On Geaster orientalis nov. spec. Grevillea 6, 39: 108–109. [Google Scholar]
- Hazslinszky FA. 1883. Bemerkungen zu den deutschen und ungarischen Geaster-Arten. Verhandlungen des Botanischen Vereins der Provinz Brandenburg und die angrenzenden Lander 24: 135–137. [Google Scholar]
- Henderson A. 2006. Traditional morphometrics in plant systematics and its role in palm systematics. Botanical Journal of the Linnean Society 151: 103–111. [Google Scholar]
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analyses. Systematic Biology 42: 182–192. [Google Scholar]
- Hollós L. 1901. Új gasteromyceta fajok magyarországból. Mathematikai és természettudományi értesítõ 19: 504–512. [Google Scholar]
- Hollós L. 1904. Die Gasteromyceten Ungarns. Weigel, Leipzig, Germany. [Google Scholar]
- Horner HT, Tiffany LH, Knaphus G. 1995. Oak-leaf-litter rhizomorphs from Iowa and Texas: Calcium oxalate producers. Mycologia 87, 1: 34–40. [Google Scholar]
- Jeppson M. 2013. Jordstjärnor. Mykologiska publikationer 6: 1–228. [Google Scholar]
- Jeppson M, Nilsson RH, Larsson E. 2013. European earthstars in Geastraceae (Geastrales, Phallomycetidae) - a systematic approach using morphology and molecular sequence data. Systematics and Biodiversity 11, 4: 437–465. [Google Scholar]
- Jiménez-Mejías P, Luceño M, Martín-Bravo S. 2014. Species boundaries within the southwest Old World populations of the Carex flava group (Cyperaceae). Systematic Botany 39, 1: 117–131. [Google Scholar]
- Kaiser HF. 1974. An index of factorial simplicity. Psychometrika 39: 31–36. [Google Scholar]
- Kasuya T, Hosaka K, Uno K, et al. 2012. Phylogenetic placement of Geastrum melanocephalum and polyphyly of Geastrum triplex. Mycoscience 53, 6: 411–426. [Google Scholar]
- Katoh K, Misawa K, Kuma K, et al. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzer A, Bruns TD. 1999. Use of atp6 in fungal phylogenetics: an example from the Boletales. Molecular Phylogenetics and Evolution 13: 483–492. [DOI] [PubMed] [Google Scholar]
- Kuhar F, Castiglia V, Papinutti L. 2013. ‘2012. ’. Geastrum species of the La Rioja province. Mycotaxon 122: 145–156. [Google Scholar]
- Lewis PO. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology 50, 6: 913–925. [DOI] [PubMed] [Google Scholar]
- Lloyd CG. 1902. The Geastrae. Bulletin of the Lloyd Library of Botany, Pharmacy and Materia Medica 2: 1–43. [Google Scholar]
- Lloyd CG. 1907. New notes on the Geasters. Mycological Writings 2: 309–317. [Google Scholar]
- Lutzoni F, Kauff F, Cox CJ, et al. 2004. Assembling the Fungal Tree of Life: progress, classification, and evolution of sucellular traits. American Journal of Botany 91, 10: 1446–1480. [DOI] [PubMed] [Google Scholar]
- Macbride TH. 1912. Notes on Iowa saprophytes - I. Geaster minimum Schw. and its relatives. Mycologia 4, 2: 84–87. [Google Scholar]
- Mann DG, Evans KM. 2007. Molecular genetics and the neglected art of diatomics. In: Brodie J, Lewis J. (eds), Unravelling the algae: the past, present and future of algal systematics: 232–265. CRC Press, USA. [Google Scholar]
- Marshall DC. 2010. Cryptic failure of partitioned Bayesian phylogenetic analyses: Lost in the land of long trees. Systematic Biology 59: 108–117. [DOI] [PubMed] [Google Scholar]
- Martín MP, Winka K. 2000. Alternative methods of extracting and amplifying DNA from lichens. Lichenologist 32, 2: 189–196. [Google Scholar]
- Massee G. 1892. British Fungus Flora 1. Clowes & Sons, London, United Kingdom. [Google Scholar]
- Matheny PB, Liu YJ, Ammirati JF, et al. 2002. Using RPB sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89, 4: 688–698. [DOI] [PubMed] [Google Scholar]
- McNeill J, Barrie FR, Buck WR, et al. 2012. International Code of Nomenclature for algae, fungi, and plants (Melbourne Code), adopted by the Eighteenth International Botanical Congress Melbourne, Australia, July 2011. Regnum Vegetabile 154. [Google Scholar]
- Medina R, Lara F, Goffinet B, et al. 2012. Integrative taxonomy successfully resolves pseudo-cryptic complex of disjunct epiphytic moss Orthotrichum consimile s.l. (Orthotrichaceae). Taxon 61: 1180–1198. [Google Scholar]
- Nilsson RH, Ryberg M, Kristiansson E, et al. 2006. Taxonomic reliability of DNA sequences in public sequence databases: a fungal perspective. PLoS ONE 1, 1: e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander JAA, Wilgenbusch JC, Warren DL, et al. 2008. AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24: 581–583. [DOI] [PubMed] [Google Scholar]
- Ochoa C, Moreno G. 2006. Hongos gasteroides y secotioides de Baja California, México. Boletín de la Sociedad Micológica de Madrid 30: 121–166. [Google Scholar]
- Olson DM, Dinerstein E, Wikramanayake ED, et al. 2001. Terrestrial ecoregions of the world: A new map of life on earth. BioScience 51: 933–938. [Google Scholar]
- Palmer JT. 1959 Observations on Gasteromycetes. VIII. Persoon's specimens of Geastrum pectinatum Pers. and a reassessment of Geastrum plicatum Berk. and G. tenuipes Berk. Persoonia 1: 149–164. [Google Scholar]
- Persoon CH. 1794. Neuer Versuch einer systematischen Einteilung der Schwämme. Neues Magazin für die Botanik 1: 63–128. [Google Scholar]
- Persoon CH. 1801. Synopsis Methodica Fungorum. Apud Henricum Dietrich, Germany. [Google Scholar]
- Pimentel RA. 1979. Morphometrics, the multivariate analysis of biological data. Kendall, Hunt Pub. Co. [Google Scholar]
- Quélet L. 1873. Les champignons des Jura et des Vosges, vol. 2. Mémoires de la Société d'émulation de Montbéliard 2, 5: 43–332. [Google Scholar]
- R Development Core Team. 2008. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org. [Google Scholar]
- Rabenhorst L. 1851. Mykologisches I. Botanische Zeitung 9: 625–629. [Google Scholar]
- Rambaut A. 2007. Molecular evolution, phylogenetics and epidemiology. FigTree v1.3. http://tree.bio.ed.ac.uk/software/figtree/. [Google Scholar]
- Rambaut A, Suchard MA, Xie D, et al. 2013. Tracer v1.5, Available from http://beast.bio.ed.ac.uk/Tracer. [Google Scholar]
- Rannala B, Zhu T, Yang Z. 2012. Tail paradox, partial identifiability and influential priors in Bayesian branch length inference. Molecular Biology and Evolution 29: 325–335. [DOI] [PubMed] [Google Scholar]
- Revelle W. 2014. psych: Procedures for Personality and Psychological Research, Northwestern University, Evanston, Illinois, USA, http://CRAN.R-project.org/package=psych. Version 1.4.4. [Google Scholar]
- Roca-Valiente B. 2013. Phylogenetic study in the Rhizocarpon geographicum group (Lichens, Rhizocarpaceae, Ascomycota). Contrasting analysis of morphological characters and biogeographic patterns. PhD thesis, Biología Vegetal II, Universidad Complutense de Madrid, Spain. [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, et al. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61, 3: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Sánchez E, Sosa V. 2010. Delimiting species boundaries within the Neotropical bamboo Otatea (Poacaeae: Bambusoideae) using molecular, morphological and ecological data. Molecular Phylogenetics and Evolution 54: 344–356. [DOI] [PubMed] [Google Scholar]
- Sáez AG, Lozano E. 2005. Body doubles. Nature 433: 111. [DOI] [PubMed] [Google Scholar]
- Sandoval-Sierra JV, Martín MP, Diéguez-Uribeondo J. 2014. Species identification in the genus Saprolegnia (Ooomycetes): Defining DNA-based molecular operational taxonomic units. Fungal Biology 118: 559–578. [DOI] [PubMed] [Google Scholar]
- Sarasini M. 2005. Gasteromiceti Epigei. Associazione Micologica Bresadola, Italy. [Google Scholar]
- Schaeffer JCh. 1763. Fungorum, qui in Bavaria et Palatinau circa Ratisbonam nascuntur icones, nativis coloribus expressae 2. Henrici Godofredi Zunkelii, Ratisbona, Germany. [Google Scholar]
- Schlick-Steiner BC, Steiner FM, Seifert B, et al. 2010. Integrative taxonomy: a multisource approach to exploring biodiversity. Annual Review of Entomology 55: 421–438. [DOI] [PubMed] [Google Scholar]
- Schmidel DCC. 1793. Icones plantarum et analyses partium aeri incisae atque vivis coloribus indicibus nominum necessariis figuram explicationibus et brevibus animadversionibus, Ed. 2. Erlangae. [Google Scholar]
- Schweinitz LD. 1822. Synopsis fungorum Carolinae superioris secundum observations. Schriften der Naturforschenden Gesellschaft zu Leipzig 1: 20–130. [Google Scholar]
- Shen Y-Y, Chen X, Murphy RW. 2013. Assessing DNA barcoding as a tool for species identification and data quality control. PLoS ONE 8, 2: e57125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons MP, Ochoterena H. 2000. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49: 369–381. [PubMed] [Google Scholar]
- Šmarda F. 1947. Nový druh hvězdice - Geaster atratus sp. n. (hvězdice černavá). Česká Mykologie 1: 71–74. [Google Scholar]
- Smith JA, Blanchette RA, Newcombe G. 2004. Molecular and morphological characterization of the willow rust fungus, Melampsora epitea, from artic and temperate hosts in North America. Mycologia 96, 6: 1330–1338. [PubMed] [Google Scholar]
- Soto MK, Wright JE. 2000. Taxonomía del género Geastrum (Basidiomycetes, Lycoperdales) en la provincia de Buenos Aires, Argentina. Boletín de la Sociedad Argentina de Botánica 34, 3–4: 185–201. [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 21: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Staněk VJ. 1958. Geastraceae. In: Pilát A (ed), Flora ČSR B1, Gasteromycetes: 392–526, 777–795. Praha. [Google Scholar]
- Stevens J. Applied multivariate statistics for the behavioral sciences (3rd ed). Mahwah, NJ, Lawrence Erlbaum. 1996 [Google Scholar]
- Stiller JW, Hall BD. 1997. The origin of red algae: implications for plastid evolution. Proceedings of the National Academy of Sciences, USA: 94: 4520–4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunhede S. 1986. Notes on the type material of Geastrum arenarium. Windhalia 15: 35–43. [Google Scholar]
- Sunhede S. 1989. Geastraceae (Basidiomycotina). Morphology, ecology and systematics with special emphasis on the North European species. Synopsis Fungorum 1: 1–534. [Google Scholar]
- Swofford DL. 2003. PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods), v. 4.0b10. Sinauer Associates, Sunderland, Massachusetts. [Google Scholar]
- Titus K, Mosher JA, Williams BK. 1984. Chance-corrected classification for use in discriminant analysis: Ecological applications. American Midland Naturalist 111: 1–7. [Google Scholar]
- Valcárcel V, Vargas P. 2010. Quantitative morphology and species delimitation under the general lineage concept: Optimization for Hedera (Araliaceae). American Journal of Botany 97, 9: 1555–1573. [DOI] [PubMed] [Google Scholar]
- Valdecasas AG, Williams D, Wheeler QD. 2008. ‘Integrative taxonomy' then and now: a response to Dayrat (2005). Biological Journal of the Linnean Society 93: 211–216. [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittadini C. 1842. Monographia Lycoperdineorum. Ex Officina Regia, Italy. [Google Scholar]
- Vondrák J, Ríha P, Arup U, et al. 2009. The taxonomy of the Caloplaca citrina group (Teloschistales) in the Black Sea region; with contributions to the cryptic species concept in lichenology. The Lichenologist 41: 571–604. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand J, Sninsky J, et al., PCR protocols: a guide to methods and applications: 315–322. Academic Press, Orlando, Florida. [Google Scholar]
- Zamora JC. 2014. Proposal to conserve the name Geastrum (Basidiomycota: Geastrales) with a conserved type. Taxon 63, 3: 679–680. [Google Scholar]
- Zamora JC, Calonge FD. 2007. Geastrum parvistriatum, una nueva especie encontrada en España. Boletín de la Sociedad Micológica de Madrid 31: 139–149. [Google Scholar]
- Zamora JC, Calonge FD, Hosaka K, et al. 2014. Systematics of the genus Geastrum (Fungi: Basidiomycota) revisited. Taxon 63, 3: 477–497. [Google Scholar]
- Zamora JC, Calonge FD, Martín MP. 2013. New sources of taxonomic information for earthstars (Geastrum, Geastraceae, Basidiomycota): phenoloxidases and rhizomorph crystals. Phytotaxa 132, 1: 1–20. [Google Scholar]
- Zhang C, Rannala B, Yang Z. 2012. Robustness of compound Dirichlet priors for Bayesian inference of branch lengths. Systematic Biology 61: 779–784. [DOI] [PubMed] [Google Scholar]
- Zwickl DJ. 2006. Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD thesis, The University of Texas at Austin, USA. [Google Scholar]