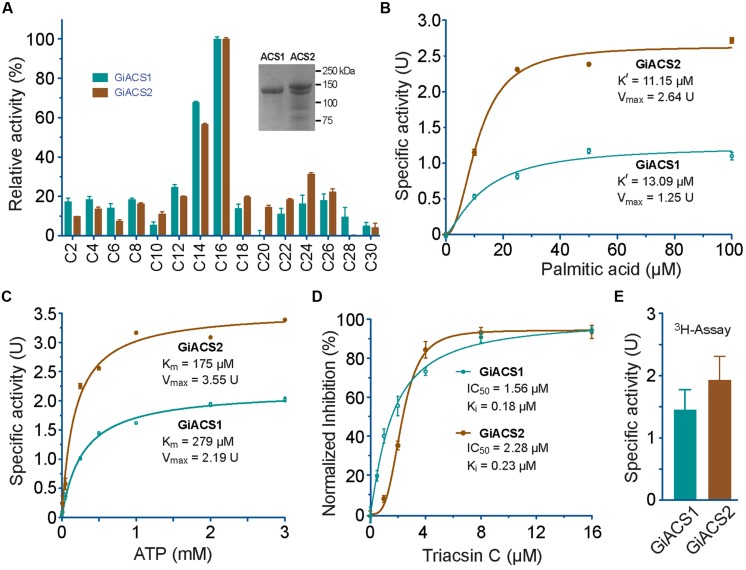
FIGURE 4.
Enzyme kinetic features of recombinant GiACS1 and GiACS2 as determined by a 5, 5′-dithio-bis-(2-nitrobenzoate) (DTNB) colorimetric assay. (A) Substrate preference of GiACS1 and GiACS2 toward FA with varied carbon chain lengths. Relative activities were shown in comparison with that on the C16:0 palmitic acid. Inset showed SDS-PAGE analysis of purified recombinant GiACS1 and GiACS2 as maltose-binding protein (MBP)-fusion proteins; (B) Allosteric kinetics of GiACS proteins on palmitic acid; (C) Michaelis–Menten kinetics of GiACS on ATP; (D) Inhibition of triacsin C on the GiACS enzyme activity; and (E) Enzyme activity by detecting the formation of 3H-palmitoyl-CoA using a heptane extraction-based radioactive assay. Bars represent SEM derived from three or more reactions. At least two independent assays were performed for each experiment, and the data from one representative assay were shown here. U = μmol/mg/min.