Fig. 4.
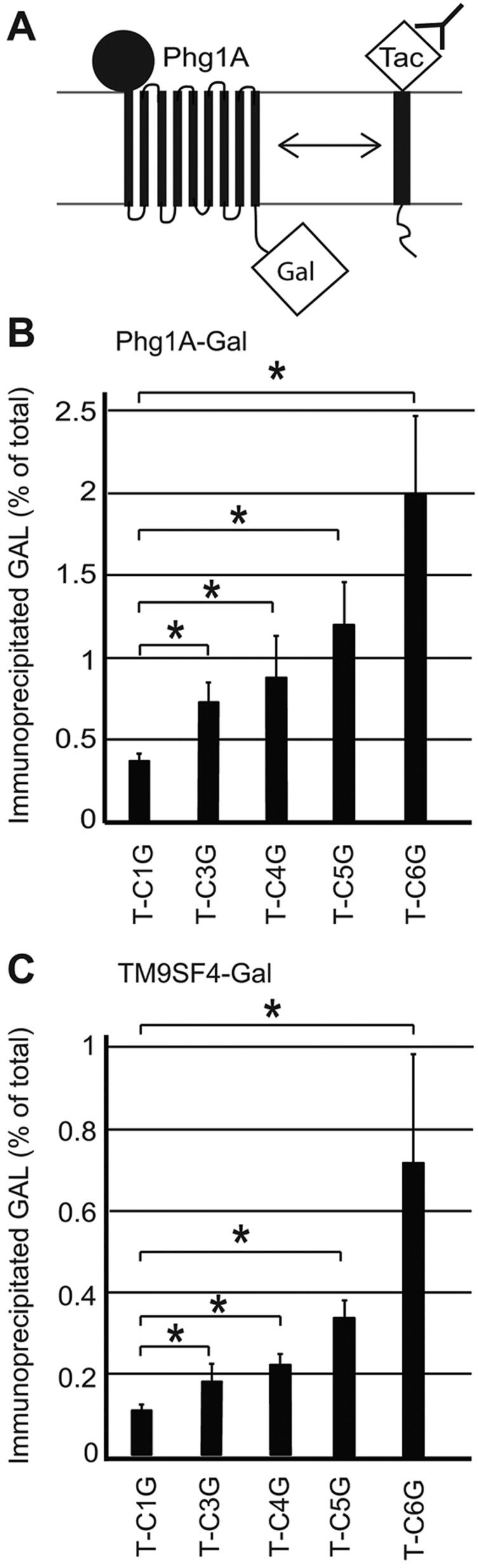
Dictyostelium Phg1A and human TM9SF4 associate preferentially with glycine-rich TMDs. (A) To reveal a putative association of Phg1A with glycine-rich TMDs, COS7 cells were co-transfected with plasmids encoding the Phg1A protein fused to β-galactosidase (Gal) and Tac fusion proteins fused to various TMDs. Tac fusion proteins were immunoprecipitated and the amount of co-precipitated β-galactosidase activity assessed to reveal the degree of association with Phg1A. (B) Phg1A–Gal was co-expressed with T-C1G (one glycine in the TMD) or with Tac mutants with three (T-C3G), four (T-C4G), five (T-C5G) or six (T-C6G) glycine residues in their TMD. Addition of glycine residues in the TMD of Tac gradually increased its interaction with Ph1A–Gal. The mean±s.e.m. of at least six experiments are indicated. (C) Interaction between human TM9SF4 and glycine-rich TMDs was determined as described in B. A specific interaction was detected between TM9SF4 and glycine-rich TMDs. The mean±s.e.m. of at least eight experiments are indicated. *P<0.05 (Student’s t-test).
