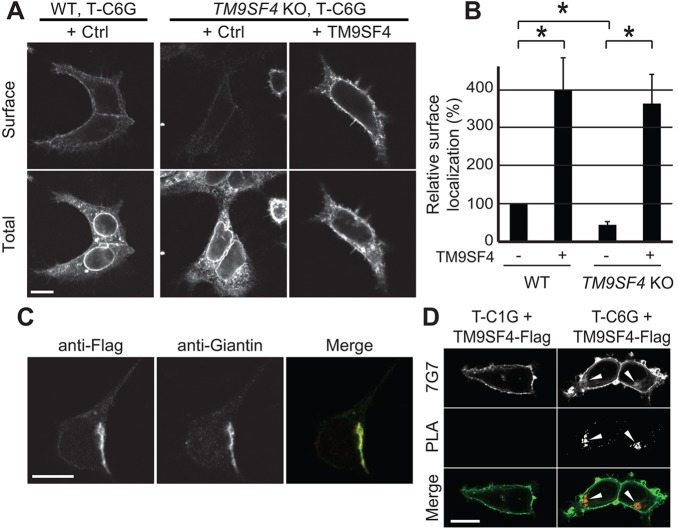
Fig. 5.
TM9SF4 controls surface localization of glycine-rich TMDs in human cells. (A) A Tac protein with six glycine residues in its TMD (T-C6G) was expressed in parental (WT) or TM9SF4 KO mammalian HEK293T cells. The protein was labeled before (Surface) and after (Total) cell permeabilization. Large amounts of T-C6G were detected in the ER in both cell types, as evidenced by the clearly visible nuclear envelope. Lower cell surface levels were observed in TM9SF4 KO cells than in parental cells. Moreover, overexpression of TM9SF4 (+TM9SF4) increased strongly cell surface levels of T-C6G. All the pictures presented here were taken sequentially with identical settings. (B) Quantification of the surface targeting of T-C6G in parental and TM9SF4 KO HEK293T cells, and in cells overexpressing TM9SF4. The mean±s.e.m. of four independent experiments are presented. *P<0.01 (Student's t-test). (C) A Flag-tagged version of TM9SF4 was expressed in HEK293T cells, and detected by immunofluorescence. It colocalized with giantin, a marker of the Golgi complex. (D) T-C1G (upper panel) or T-C6G (lower panel) were co-expressed with a Flag-tagged version of TM9SF4 in HEK293T WT cells. A proximity ligation assay (PLA) revealed a specific signal in the Golgi complex when T-C6G and TM9SF4–Flag were co-expressed (arrowheads). Scale bars: 10 µm.