Fig. 1.
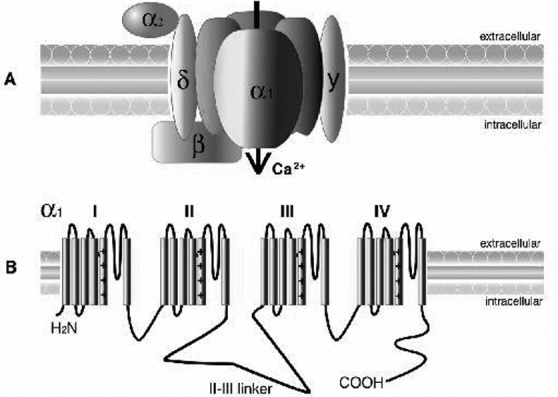
A) Calcium channel structure comprising the α1, α2δ, β, and, γ subunits. The α1 subunit, represented here with its 4-fold monomeric structure, is responsible for many of the functional characteristics of these channels, including the pore voltagedependent gating and dihydropyridine binding. The α2 is the extracellular glycosylated subunit that interacts with the α1 subunit. The δ subunit has a single transmembrane region with a short intracellular portion that serves to anchor the protein in the plasma membrane. The β subunit is the only Ca2+ channel subunit that is entirely cytoplasmic. The γ1 subunit is a glycoprotein that, for the most part, is not required to regulate the channel complex. B) The α1 subunit forms the Ca2+ selective pore, which contains voltage-sensing apparatus and drug/toxin-binding sites. This subunit contains 4 homologous domains (labelled I–IV), each containing 6 transmembrane helices (S1–S6).
