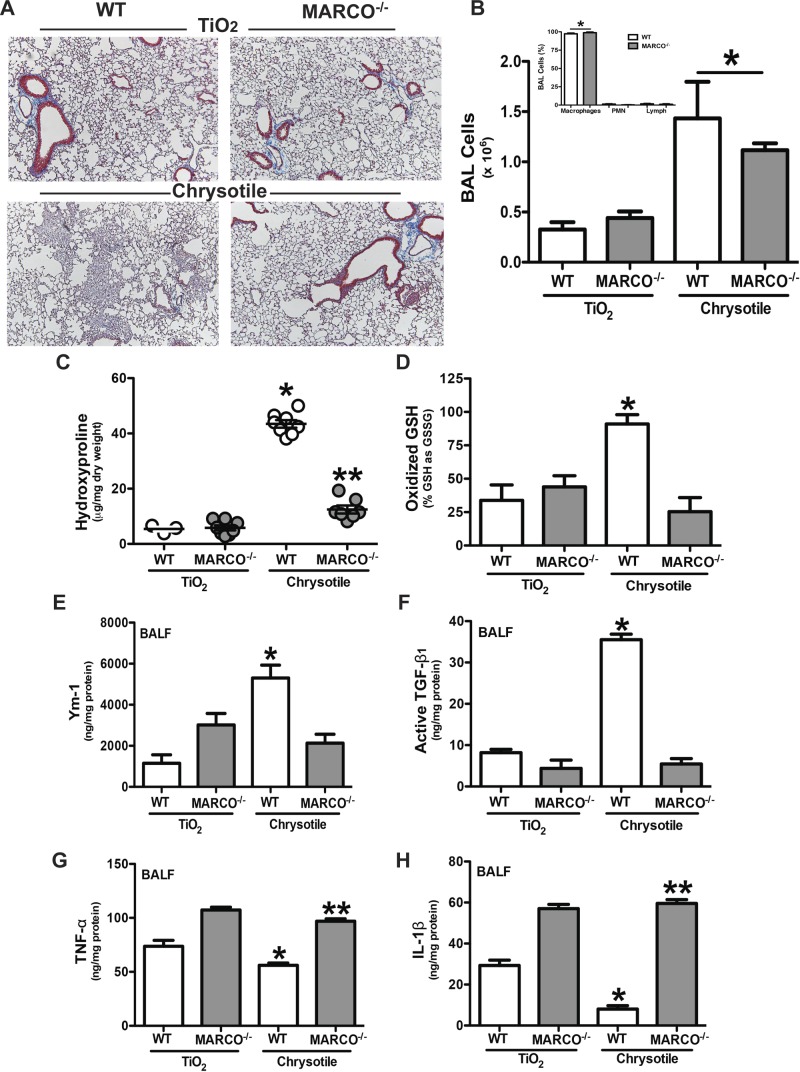
Figure 3.
MARCO is required for the development of pulmonary fibrosis. WT and MARCO−/− mice were intratracheally administered 100 μg/mouse of TiO2 or chrysotile asbestos. Twenty-one days later, mice were killed, BAL was performed, and lungs were removed. A) Lungs were stained for collagen with Masson’s trichrome stain. Representative sections from WT (n = 4) and MARCO−/− (n = 4) mice are shown. B) Cells obtained by BAL from WT (n = 5, TiO2; n = 6, chrysotile) and MARCO−/− (n = 5, TiO2; n = 7, chrysotile) mice. *P < 0.0001 vs. TiO2-exposed mice. (Inset) Cell differential from chrysotile-exposed mice was determined by Wright-Giemsa stain. *P < 0.0001 (n = 4) macrophages vs. all other cell types. C) Hydroxyproline content in lungs is shown as microgram hydroxyproline per milligram lung dry weight. *P < 0.0001 vs. all other groups, and **P < 0.0014 vs. TiO2-exposed mice. WT (n = 3, TiO2; n = 8, chrysotile) and MARCO−/− (n = 8, TiO2; n = 7, chrysotile) mice. D) GSH and GSSG were measured by HPLC and mass spectrometry analysis and are expressed as oxidized glutathione (GSSG) relative to its reduced form (GSH). *P < 0.05. WT (n = 6) and MARCO−/− (n = 7, TiO2; n = 6, chrysotile) mice. BALF was obtained by BAL. ELISA was performed for (E) Ym-1, *P < 0.0002 vs. all other groups, WT (n = 5, TiO2; n = 8, chrysotile) and MARCO−/− (n = 7, TiO2; n = 8, chrysotile); (F) active TGF-β1, *P < 0.0001 vs. all other groups; (G) TNF-α, *P < 0.0001 vs. all other groups and **P < 0.0009 vs. WT groups; and (H) IL-1β, * P < 0.0001 vs. all other groups and **P < 0.0001 vs. WT groups. WT (n = 4, TiO2; n = 8, chrysotile) and MARCO−/− (n = 5, TiO2; n = 8, chrysotile) (F–H).