Figure 2.
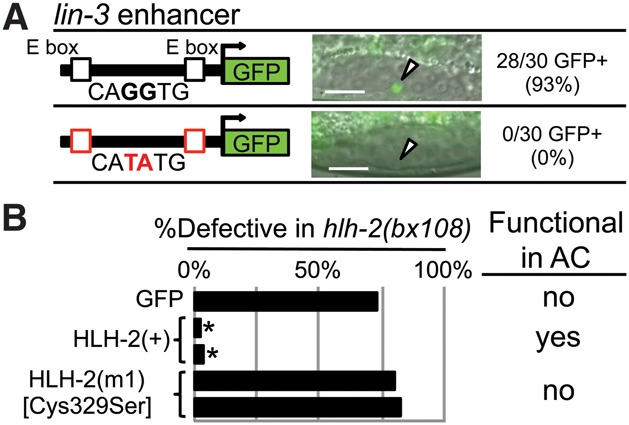
Assessing HLH-2 function as a homodimer in the AC. As shown in Supplemental Figures S2–S5, we analyzed the expression of 17 class II bHLH genes, potential heterodimerization partners of HLH-2; none were expressed in the α or β cells, the AC, or the VUs. In addition, we analyzed the function of all 42 C. elegans bHLH genes (the class II genes plus additional classes) by performing RNAi in a background sensitized for RNAi and for detection of a potential HLH-2 heterodimerization partner and saw no effect on AC number or function. These analyses suggested that HLH-2 functions as a homodimer in the AC, further tested here. (A) In ACEL, two homodimer-binding E boxes, CAGGTG, promote expression in the AC; mutation of these E boxes to the functional heterodimer-binding type CATATG abolishes expression in the AC. n = 30 for each of three transgenic lines; see Supplemental Figure S7 for details. An arrowhead marks the AC. Bar, 10 μm. (B) Function of HLH-2 mutants was assessed by rescue of abnormal vulval eversion (Evl), reflecting abnormal AC function, caused by hlh-2(bx108); details are in Supplemental Figure S8. GFP-HLH-2 has rescuing activity, whereas GFP-HLH-2(C329S) [HLH(m1)], predicted to have disrupted homodimerization, does not. (*) P < 0.0001 by two-tailed Fisher's exact test compared with GFP alone.
