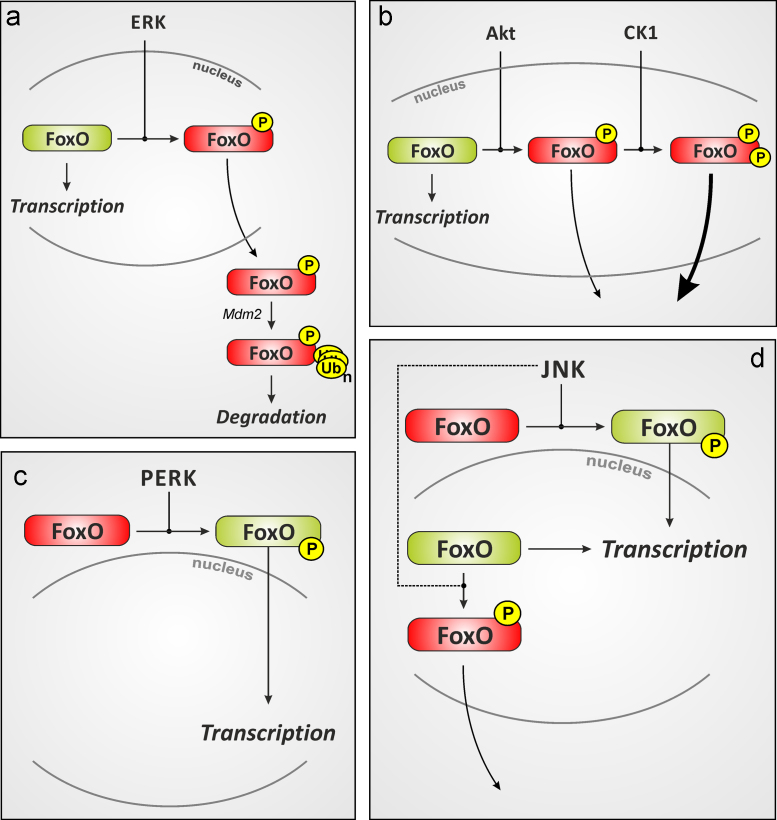
Fig. 4.
FoxO phosphorylation and its biological consequences. Schematic representation of FoxO phosphorylation by different kinases and the consequences with respect to activity and subcellular localization. (A) ERK-catalyzed phosphorylation of FoxOs may cause nuclear exclusion and murine double-minute (Mdm)-2-dependent proteasomal degradation [65]. Similarly, Akt (B) catalyzed FoxO phosphorylation will cause FoxO inactivation, nuclear exclusion and may trigger FoxO degradation (not shown). Interestingly, FoxO1a phosphorylation at S319 was shown to prime for a consecutive phosphorylation by casein kinase 1 (CK1), which further enhances nuclear exclusion [319]. FoxO phosphorylation may also result in nuclear accumulation and activation: (C) ER-stress may cause PERK-dependent FoxO phosphorylation and activation [244]. (D) c-Jun-N-terminal kinase (JNK)-dependent FoxO phosphorylation was described as activating (FoxO4 [93]) or inactivating (FoxO3a; dashed lines [317]). Phosphorylation is indicated by a black “P” on yellow background and stands for phosphorylations at multiple different sites (e.g. Akt: T24, S256, S319 for human FoxO1a). See Table 1 for further explanations. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)