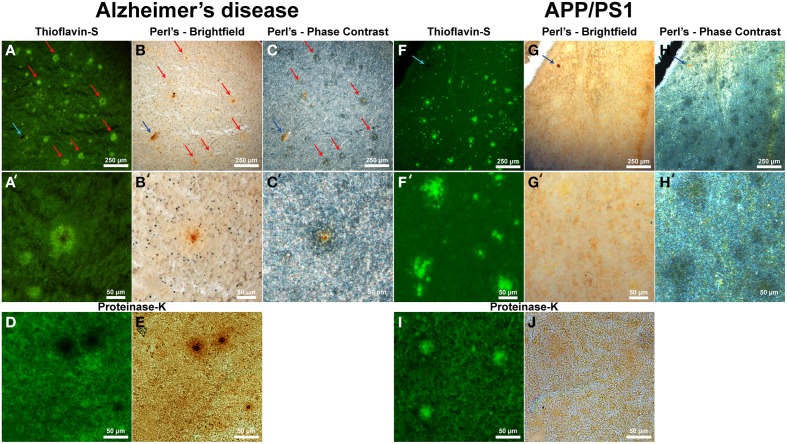
Figure 5.
Alzheimer's disease (A–E) and APP/PS1 (F–J) tissue stained with thioflavin-S and a traditional Perl's stain under fluorescence (left), bright-field (middle), and phase contrast (right) microscopy at 40x (A–J) and 200x (A′–H′) magnification. Red arrows indicate selected plaques that have ferric iron associated with them, while the blue arrow indicates a focal iron region not associated with an Aβ plaque and is composed of hemosiderin or magnetite. The core of numerous dense core Aβ plaques in the AD tissue samples exhibit focal iron deposition and diffuse iron throughout their coronal regions in both the phase contrast and bright-field image sets. The APP/PS1 transgenic mouse tissue does not stain positive for focal iron associated with fibrillar Aβ plaques with the Perl's stain. The blue arrow illustrates a focal iron deposit not associated with a plaque that indicates the Perl's stain is effective in staining ferric iron deposits. Phase contrast microscopy shows regions where fibrillar thioflavin-s positive Aβ plaques are located, as well as thioflavin-s negative regions beyond the fibrillar deposition. The modified thioflavin-S and Perl's stain use protein digestion to allow further penetration of the aqueous stain into the hydrophobic AD (D,E) and APP/PS1 (I,J) plaques. The stain indicates that there is a very minute amount of iron found in the APP/PS1 plaques (J) that is not stainable with traditional Perl's staining methodology. Human plaques no longer stain positive for thioflavin-S (D) due to Aβ protein degradation and subsequent lack of thioflavin-S Aβ fibril intercalation, however the morphology of the ferric iron staining verifies plaque location. There is a large visual amount of iron in the core and halo region of the AD tissue (E) that is not seen in the transgenic tissue. Scale bars are 250 and 50 μm.